TAVALISSE
These highlights do not include all the information needed to use TAVALISSE safely and effectively. See full prescribing information for TAVALISSE. TAVALISSE (fostamatinib disodium hexahydrate) tablets, for oral useInitial U.S. Approval: 2018
21149cc3-049b-43e2-b141-c9499160556c
HUMAN PRESCRIPTION DRUG LABEL
Jan 2, 2024
Rigel Pharmaceuticals, Inc.
DUNS: 967965468
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
FOSTAMATINIB
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
FOSTAMATINIB
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle Label
NDC 71332-002-01
Rx only
Tavalisse®
(fostamatinib) tablets
150 mg
60 Tablets

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically important adverse reactions, that can become serious are described elsewhere in the labeling:
- Hypertension [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Diarrhea [see Warnings and Precautions (5.3)]
- Neutropenia [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
TAVALISSE was studied in two randomized, double-blind, placebo-controlled trials that were identical in design. The data described below reflect exposure to TAVALISSE in 102 patients with chronic ITP who had received one or more prior ITP treatment(s). Groups were stratified with respect to splenectomy and severity of thrombocytopenia. Patients randomized to the TAVALISSE arm received 100 mg orally twice daily. Based upon platelet count and tolerability, if a patient's platelet count did not increase to at least 50 × 109/L, the TAVALISSE dose could be increased to 150 mg twice daily after one month. In the placebo controlled studies, the median duration of TAVALISSE exposure in these studies was 86 days (range 8 to 183) [see Clinical Studies (14) for additional details for patients on TAVALISSE].
In the ITP double-blind studies, serious adverse drug reactions were febrile neutropenia, diarrhea, pneumonia, and hypertensive crisis, which each occurred in 1% of patients receiving TAVALISSE. In addition, severe adverse reactions observed in patients receiving TAVALISSE included dyspnea and hypertension (both 2%); and neutropenia, arthralgia, chest pain, diarrhea, dizziness, nephrolithiasis, pain in extremity, toothache, syncope and hypoxia (all 1%) [see Warnings and Precautions (5.1)]. Table 3 presents the common adverse reactions from these studies.
Table 3: Incidence of Common (≥ 5%) Adverse Reactions from Double- Blind Clinical Studies (FIT 1 and FIT 2)|
Adverse Reaction |
TAVALISSE |
Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
|
Mild |
Moderate |
Severe |
TOTAL |
Mild |
Moderate |
Severe |
TOTAL | |
|
ALT = Alanine aminotransferase | ||||||||
| ||||||||
|
Diarrhea* |
21 |
10 |
1 |
31 |
13 |
2 |
0 |
15 |
|
Hypertension† |
17 |
9 |
2 |
28 |
10 |
0 |
2 |
13 |
|
Nausea |
16 |
3 |
0 |
19 |
8 |
0 |
0 |
8 |
|
Dizziness |
8 |
2 |
1 |
11 |
6 |
2 |
0 |
8 |
|
ALT increased |
5 |
6 |
0 |
11 |
0 |
0 |
0 |
0 |
|
AST increased |
5 |
4 |
0 |
9 |
0 |
0 |
0 |
0 |
|
Respiratory infection‡ |
7 |
4 |
0 |
11 |
6 |
0 |
0 |
6 |
|
Rash§ |
8 |
1 |
0 |
9 |
2 |
0 |
0 |
2 |
|
Abdominal pain¶ |
5 |
1 |
0 |
6 |
2 |
0 |
0 |
2 |
|
Fatigue |
4 |
2 |
0 |
6 |
0 |
2 |
0 |
2 |
|
Chest pain |
2 |
3 |
1 |
6 |
2 |
0 |
0 |
2 |
|
Neutropenia# |
3 |
2 |
1 |
6 |
0 |
0 |
0 |
0 |
|
Enzyme |
Maximum Level of Elevation |
Number of Patients (%) | |
|---|---|---|---|
|
TAVALISSE |
Placebo | ||
|
Alanine aminotransferase (ALT) and/or Aspartate aminotransferase (AST) |
|
3 (3) |
0 |
|
5 (5) |
0 | |
|
≥10 × ULN |
1 (1) |
0 |
The most common adverse reactions (≥5% and more than placebo) are diarrhea, hypertension, nausea, respiratory infection, dizziness, ALT/AST increased, rash, abdominal pain, fatigue, chest pain and neutropenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rigel Pharmaceuticals, Inc. at 1-800-983-1329 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TAVALISSE
Strong CYP3A4 Inhibitors
Concomitant use with strong CYP3A4 inhibitors increases exposure to R406 (the major active metabolite), which may increase the risk of adverse reactions. Monitor for toxicities of TAVALISSE that may require dose reduction (see Table
- when given concurrently with a strong CYP3A4 inhibitor [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Strong CYP3A4 Inducers
Concomitant use with a strong CYP3A4 inducer reduces exposure to R406. Concomitant use of TAVALISSE with strong CYP3A4 inducers is not recommended [see Clinical Pharmacology (12.3)].
7.2 Effect of TAVALISSE on Other Drugs
CYP3A4 Substrates
Concomitant use of TAVALISSE may increase concentrations of some CYP3A4 substrate drugs. Monitor for toxicities of CYP3A4 substrate drug that may require dosage reduction when given concurrently with TAVALISSE [see Clinical Pharmacology (12.3)].
BCRP Substrates
Concomitant use of TAVALISSE may increase concentrations of BCRP substrate drugs (e.g., rosuvastatin). Monitor for toxicities of BCRP substrate drug that may require dosage reduction when given concurrently with TAVALISSE [see Clinical Pharmacology (12.3)].
P-Glycoprotein (P-gp) Substrates
Concomitant use of TAVALISSE may increase concentrations of P-gp substrates (e.g., digoxin). Monitor for toxicities of the P-gp substrate drug that may require dosage reduction when given concurrently with TAVALISSE [see Clinical Pharmacology (12.3)].
- Strong CYP3A4 Inhibitors: Concomitant use with a strong CYP3A4 inhibitor increases exposure to R406 (the major active metabolite).(7)
- Strong CYP3A4 Inducers: Concomitant use is not recommended. (7)
DESCRIPTION SECTION
11 DESCRIPTION
Fostamatinib is a tyrosine kinase inhibitor. TAVALISSE is formulated with the disodium hexahydrate salt of fostamatinib, a phosphate prodrug that converts to its pharmacologically active metabolite, R406, in vivo.
The chemical name for fostamatinib disodium hexahydrate is disodium (6-[[5-fluoro-2-(3,4,5-trimethoxyanilino) pyrimidin-4-yl]amino]-2,2-dimethyl-3-oxo-pyrido[3,2-b][1,4]oxazin-4-yl)methyl phosphate hexahydrate. The molecular formula is C23H24FN6Na2O9P∙6H2O, and the molecular weight is 732.52. The structural formula is:
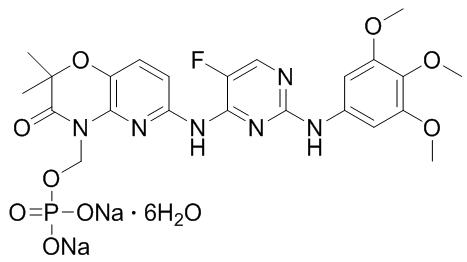
Fostamatinib disodium is a white to off-white powder that is practically insoluble in pH 1.2 aqueous buffer, slightly soluble in water, and soluble in methanol.
Each TAVALISSE oral tablet contains 100 mg or 150 mg fostamatinib, equivalent to 126.2 mg or 189.3 mg fostamatinib disodium hexahydrate, respectively.
The inactive ingredients in the tablet core are mannitol, sodium bicarbonate, sodium starch glycolate, povidone, and magnesium stearate. The inactive ingredients in the film coating are polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc, iron oxide yellow, and iron oxide red.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fostamatinib is a tyrosine kinase inhibitor with demonstrated activity against spleen tyrosine kinase (SYK). The major metabolite of fostamatinib, R406, inhibits signal transduction of Fc-activating receptors and B-cell receptor. The fostamatinib metabolite R406 reduces antibody-mediated destruction of platelets.
12.2 Pharmacodynamics
Mean treatment-related increases of 2.93 mmHg in systolic blood pressure and 3.53 mmHg in diastolic blood pressure over placebo were observed following TAVALISSE doses of 100 mg twice daily for 28 days. About 31% of patients in the TAVALISSE group experienced blood pressures ≥140/90 mmHg compared to 15% of patients in the placebo group. Blood pressure returned to baseline within 1 week following TAVALISSE discontinuation in 58% (11 of 19) of patients in the TAVALISSE group who had blood pressures ≥140/90 mmHg.
Cardiac Electrophysiology
At 2 times the maximum recommended dose, TAVALISSE did not prolong the QT interval to a clinically relevant extent.
12.3 Pharmacokinetics
TAVALISSE is a prodrug that is converted in the gut to the major active metabolite, R406. Mean (± standard deviation [SD]) exposure estimates of R406 are 550 (± 270) ng/mL for Cmax and 7080 (± 2670) ng∙h/mL for AUC. R406 exposure is approximately dose proportional up to 200 mg twice daily (1.3 times the 150 mg dosage). R406 accumulates approximately 2- to 3-fold upon twice daily dosing at 100–160 mg (0.67 to 1.06 times the 150 mg dosage).
Absorption
After oral administration of TAVALISSE, the absolute bioavailability of R406 was 55%. The median tmax of R406 is approximately 1.5 hours (range: 1 to 4 hours). Negligible levels of fostamatinib were found in plasma.
Effect of Food
Administration of TAVALISSE with a high-calorie, high-fat meal (deriving approximately 150, 250, and 500–600 calories from protein, carbohydrate, and fat, respectively) increased R406 AUC by 23% and Cmax by 15% [see Dosage and Administration (2.1)].
Distribution
In in vitro studies, the R406 is 98.3% protein bound in human plasma. The red blood cell to plasma concentration ratio is approximately 2.6. The mean (± SD) volume of distribution at steady-state of R406 is 256 (± 92) L.
Elimination
The mean (± SD) terminal half-life of R406 is approximately 15 (± 4.3) hours.
Metabolism
TAVALISSE is metabolized in the gut by alkaline phosphatase to the major active metabolite, R406. R406 is extensively metabolized, primarily through pathways of CYP450-mediated oxidation (by CYP3A4) and glucuronidation (by UDP glucuronosyltransferase [UGT]1A9). R406 is the predominant moiety in the systemic circulation, and there was minimal exposure to any R406 metabolites.
Excretion
Following an oral dose of TAVALISSE, approximately 80% of the R406 metabolite is excreted in feces with approximately 20% excreted in the urine. The major component excreted in urine was R406 N-glucuronide. The major components excreted in feces were R406, O-desmethyl R406 and a metabolite produced by gut bacteria from the O-desmethyl metabolite of R406.
Specific Populations
Population pharmacokinetics analyses indicate TAVALISSE is not altered based on age, sex, race/ethnicity. In addition, the pharmacokinetics of TAVALISSE is not altered in patients with renal impairment (creatinine clearance [CLcr] ≥ 30 to < 50 mL/min, estimated by Cockcroft Gault equation and end stage renal disease requiring dialysis), or hepatic impairment (Child-Pugh Class A, B and C).
Drug Interaction Studies
Clinical Pharmacology Studies
No significant interactions were seen with concomitant use of TAVALISSE with the following drugs: methotrexate (OAT1/3 transporters), midazolam (CYP3A4 substrate), microgynon (ethinyl estradiol and levonorgestrel), warfarin, pioglitazone (CYP2C8 substrate) and ranitidine (H2-antagonist that increases gastric pH).
Effect of Other Drugs on TAVALISSE
Strong CYP3A4 inhibitor: Concomitant use of ketoconazole (200 mg twice daily for 3.5 days) with a single dose of 80 mg TAVALISSE (0.53 times the 150 mg dosage) increased R406 AUC by 102% and Cmax by 37%.
Moderate CYP3A4 Inhibitor: Concomitant use of verapamil (80 mg three times daily for 4 days) with a single dose of 150 mg TAVALISSE increased R406 AUC by 39% and Cmax by 6% .
CYP3A4 inducer: Concomitant use of rifampicin (600 mg once daily for 8 days) with a single dose of 150 mg TAVALISSE decreased R406 AUC by 75% and Cmax by 59% .
Effect of TAVALISSE on Other Drugs
CYP3A4 substrate: Concomitant use of simvastatin (single dose 40 mg) with 100 mg twice daily TAVALISSE increased simvastatin AUC by 64% and Cmax by 113% and simvastatin acid AUC by 64% and Cmax by 83%.
BCRP substrate: Concomitant use of rosuvastatin (single dose 20 mg) with 100 mg twice daily TAVALISSE increased rosuvastatin AUC by 95% and Cmax by 88%.
P-gp substrate: Concomitant use of digoxin (0.25 mg once daily) with 100 mg twice daily TAVALISSE increased digoxin AUC by 37% and Cmax by 70% .
In Vitro Studies
TAVALISSE is an inhibitor of the human P-gp efflux transporter in vitro.
CYP3A4 and UGT1A9 are involved in the metabolism of R406. R406 is a substrate of P-gp but not of other major transporters (OAT1/3, OCT2, OATP1B1/3, MRP2, and BCRP). R406 can inhibit CYP3A4 and BCRP, and can induce CYP2C8 activity.
R406 is an inhibitor of UGT1A1. Inhibition of UGT1A1 may result in increased unconjugated bilirubin in the absence of other LFT abnormalities.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
TAVALISSE 100 mg tablets are round, biconvex, orange, film-coated tablets debossed with "100" on one side and "R" on the reverse side.
TAVALISSE 150 mg tablets are oval, biconvex, orange, film-coated tablets debossed with "150" on one side and "R" on the reverse side.
|
100 mg tablets: Available in bottle of 60 with 2 desiccant canisters |
NDC 71332-001-01 |
|
150 mg tablets: Available in bottle of 60 with 2 desiccant canisters |
NDC 71332-002-01 |
Store at room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not remove desiccants.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fostamatinib was not carcinogenic in a 2-year study in mice when administered daily by oral gavage at doses up to 500/250 mg/kg/day, and was not carcinogenic in rats when administered by oral gavage at 45 mg/kg/day.
Fostamatinib and its major active metabolite (R406) were not mutagenic in an in vitro bacterial reverse mutation (Ames) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or an in vivo mouse bone marrow micronucleus assay.
In a fertility study with oral fostamatinib, all mating (e.g., time to mating, breeding proficiency), sperm assessments (e.g., number and motility), and organ weight (e.g., paired testis weight) parameters in male rats were unaffected by dosages as high as 40 mg/kg/day, which is 6.7 times the MRHD. All mating and fertility parameters in female rats were unaffected by dosages as high as 11 mg/kg/day (which is 1.8 times the MRHD), but a slight decrease in pregnancy rates and an increase in post-implantation loss were seen at 25 mg/kg/day, which is 4.2 times the MRHD.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
TAVALISSE was studied in two placebo-controlled efficacy and safety studies (referred to as FIT-1 [NCT02076399] and FIT-2 [NCT02076412]), and in an open- label extension study referred to as FIT-3 (NCT 02077192).
Randomized, Placebo-Controlled Studies
A total of 150 patients with persistent or chronic ITP, who had an insufficient response to previous treatment (which included corticosteroids, immunoglobulins, splenectomy, and/or a thrombopoietin receptor agonists) were enrolled in two identical, double-blind, placebo-controlled studies that were conducted in different countries. For each study, patients were randomized 2:1 to TAVALISSE or placebo for 24 weeks; randomization was stratified with respect to prior splenectomy and severity of thrombocytopenia. Stable concurrent ITP therapy (glucocorticoids [< 20 mg prednisone equivalent per day], azathioprine, or danazol) was allowed, and rescue therapy was permitted, if needed. All patients initially received study drug at 100 mg twice daily (or matching placebo). Based on platelet count and tolerability, dose escalation to 150 mg twice daily (or matching placebo) was undertaken in 88% of patients at Week 4 or later. Patients who did not respond to treatment after 12 weeks, as well as patients who completed the 24-week double blind study, were eligible to enroll in open-label extension study (FIT-3).
Patients enrolled in the placebo-controlled studies had a median age of 54 years (range: 20 to 88), and the majority were female (61%) and were White (93%). Prior ITP treatments were varied, with the most common including corticosteroids (94%), immunoglobulins (53%), and thrombopoietin receptor agonists (TPO-RA) (48%). Most patients had chronic ITP (93%), with a median time since ITP diagnosis of 8.45 years, and 35% had undergone splenectomy. At baseline, the median platelet count was 16 × 109/L (with almost half [45]%) less than 15 × 109/L) and 47% were on stable ITP therapy.
In Study FIT-1, 76 patients were randomized; 51 to the TAVALISSE group and 25 to the placebo group. In Study FIT-2, 74 patients were randomized; 50 to the TAVALISSE group and 24 to the placebo group. The efficacy of TAVALISSE was based on stable platelet response (at least 50 ×109/L on at least 4 of the 6 visits between Weeks 14 to 24). Study outcomes for FIT-1 and FIT-2 are shown in Table 5.
Table 5: Study Outcomes from Placebo-Controlled Clinical Studies|
Study Outcomes |
Study FIT-1 |
Study FIT-2 | ||
|---|---|---|---|---|
|
TAVALISSE |
Placebo |
TAVALISSE |
Placebo | |
|
n (%) |
n (%) |
n (%) |
n (%) | |
|
NS = Did not demonstrate a stastistically significant difference between treatment arms | ||||
| ||||
|
Stable platelet response*,† |
9 (18) |
0 (0) |
8 (16) |
1 (4) |
|
p‡ = 0.03 |
NS | |||
|
Rolled-over into FIT-3 at Week 12§ |
28 (55) |
22 (88) |
33 (66) |
19 (79) |
|
Completed study (Week 24) |
12 (24) |
1 (4) |
13 (26) |
2 (8) |
In the FIT-1 and FIT-2 studies a total of 47 patients in the TAVALISSE arm had received a prior TPO-RA treatment; among these patients, 8 patients (17%) achieved a stable response to TAVALISSE. All 8 patients had previously discontinued TPO-RA due to loss of effect. Rescue medication was required by 30% and 45% of patients receiving TAVALISSE or placebo, respectively.
During the placebo-controlled studies, the incidence of bleeding occurred in 29% and 37% of patients in the TAVALISSE and placebo arms, respectively. Moderate, severe and serious bleeding events are described in Table 6. All severe events led to hospitalizations.
Table 6: Incidence of Moderate, Severe and Serious Bleeding-Related Events (Placebo-Controlled Efficacy Population)|
Parameter |
TAVALISSE |
Placebo |
|---|---|---|
|
Incidence of moderate bleeding-related adverse events |
9 (9) |
5 (10) |
|
Incidence of severe bleeding-related adverse events |
1 (1) |
3 (6) |
|
Incidence of serious bleeding-related adverse events |
4 (4) |
5 (10) |
Extension Study
The FIT-3 trial is an open label extension study. Patients from FIT-1 and FIT-2 who completed 24 weeks of treatment, or who did not respond to treatment any time after 12 weeks, were eligible to enroll in this study. Patients remained blinded to their treatment assignment from the previous study (TAVALISSE or placebo), so their starting dose in this study was based on their final platelet count. Patients designated as responders (defined as achievement of platelet count of at least 50 × 109/L) at the time of roll over continued in the extension study at their current trial dose and regimen. Patients who entered the extension study as non-responders (defined as platelet count less than 50 × 109/L) received TAVALISSE 100 mg twice daily regardless of their dose and regimen in the prior study.
For the FIT-3 trial, 123 patients were enrolled, 44 patients previously randomized to placebo and 79 patients previously randomized to TAVALISSE. Stable response in this study was prospectively defined as no 2 visits, at least 4 weeks apart, with a platelet count less than 50 × 109/L, without an intervening visit with a platelet count of at least 50 × 109/L (unrelated to rescue therapy), within a period of 12 weeks following initial achievement of the target platelet count. Sixty-one of the 123 subjects (50%) have discontinued from the study early.
In a prospectively defined analysis, the 44 subjects treated with placebo in the prior study were evaluated for stable response for TAVALISSE. Ten of these subjects (23%) (including a single subject who was classified as a placebo responder in the prior study) met the criteria for stable response.
Among the subjects who achieved stable response in FIT-1, FIT-2 and FIT-3 trials, 18 subjects maintained the platelet count of at least 50 × 109/L for 12 months or longer.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
-
Hypertension:
Inform patients that periodic monitoring of their blood pressure is required, as high blood pressure has occurred in patients taking TAVALISSE. Inform patients of the signs and symptoms of hypertension. Advise patients to undergo routine blood pressure monitoring and to contact their health care provider if blood pressure is elevated or if they experience signs or symptoms of hypertension [see Warnings and Precautions (5.1)]. -
Hepatotoxicity:
Inform patients that periodic monitoring of their liver enzymes is required, and any elevations (which may indicate liver injury) will be managed appropriately, including interruption, reduction, or discontinuation of TAVALISSE [see Warnings and Precautions (5.2)]. -
Diarrhea:
Advise patients to use supportive care measures, and if diarrhea becomes severe, it may necessitate interruption, reduction, or discontinuation of TAVALISSE [see Warnings and Precautions (5.3)]. -
Neutropenia:
Inform patients that monitoring of their complete blood counts is required, and a decrease in neutrophils may necessitate interruption, reduction, or discontinuation of TAVALISSE [see Warnings and Precautions (5.4)]. -
Advise patients to inform their healthcare providers of all their medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)].
-
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the potential risk to a fetus [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after receiving the last dose of TAVALISSE [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1, 8.3)]. -
Lactation
Advise lactating women not to breastfeed during treatment with TAVALISSE and for at least 1 month after the last dose [see Use in Specific Populations (8.2)]. -
Inform patients that TAVALISSE may be taken with or without food. In the case of a missed dose of TAVALISSE, instruct patients to take their next dose at its regularly scheduled time.
SPL PATIENT PACKAGE INSERT SECTION
|
PATIENT INFORMATION | |
|---|---|
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Issued: November 2020 |
|
What is the most important information I should know about TAVALISSE? *High blood pressure (hypertension). New or worsening high blood pressure is common in people treated with TAVALISSE and can be severe. Your healthcare provider will check your blood pressure regularly during treatment with TAVALISSE. If needed, your healthcare provider may start you on blood pressure medicine or change your current medicine to treat your blood pressure. Tell your healthcare provider if you get headaches, confusion, dizziness, chest pain or shortness of breath. *Liver problems. Changes in liver function blood tests are common with TAVALISSE. Liver problems may occur and can be severe. Your healthcare provider will regularly do blood tests to check how well your liver is working during treatment with TAVALISSE. *Diarrhea. Diarrhea is common in people treated with TAVALISSE and can be severe. Tell your healthcare provider if you get diarrhea during treatment with TAVALISSE. Your healthcare provider may recommend changes in your diet, drinking more water, or medicine to limit these symptoms. *Decrease in white blood cell counts (neutropenia). Decreases in your white blood cell count are common with TAVALISSE and can be severe. This may increase your risk for infection, including serious infections. Your healthcare provider will regularly do blood tests to check your white blood cell counts. Your healthcare provider may change your dose, temporarily stop, or
permanently stop treatment with TAVALISSE if you have side effects. | |
|
What is TAVALISSE? | |
|
Before you take TAVALISSE, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking TAVALISSE with certain other medicines may affect how the other
medicines work and other medicines may affect how TAVALISSE works. | |
|
How should I take TAVALISSE?
| |
|
What are the possible side effects of TAVALISSE? | |
|
|
|
These are not all the side effects of TAVALISSE. For more information, ask
your healthcare provider or pharmacist. | |
|
How should I store TAVALISSE?
Keep TAVALISSE and all medicines out of the reach of children. | |
|
General information about the safe and effective use of TAVALISSE | |
|
What are the ingredients in TAVALISSE? |
