Products (1)
Glipizide and Metformin Hydrochloride
70518-0373
ANDA077270
ANDA (C73584)
ORAL
January 5, 2024
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Glipizide and Metformin Hydrochloride
GENERIC: Glipizide and Metformin Hydrochloride
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 70518-0373-0
NDC: 70518-0373-1
COLOR: pink
SHAPE: OVAL
SCORE: No score
SIZE: 17 mm
IMPRINT: 93;7457
PACKAGING: 90 in 1 BOTTLE, PLASTIC
PACKAGING: 180 in 1 BOTTLE, PLASTIC
ACTIVE INGREDIENT(S):
- METFORMIN HYDROCHLORIDE 500mg in 1
- GLIPIZIDE 5mg in 1
INACTIVE INGREDIENT(S):
- STARCH, CORN
- CROSCARMELLOSE SODIUM
- MAGNESIUM STEARATE
- CELLULOSE, MICROCRYSTALLINE
- POLYETHYLENE GLYCOL 3350
- POLYETHYLENE GLYCOL 4000
- POLYVINYL ALCOHOL, UNSPECIFIED
- POVIDONE K30
- POVIDONE K90
- TALC
- TITANIUM DIOXIDE
- FERROSOFERRIC OXIDE
- FERRIC OXIDE RED
- FERRIC OXIDE YELLOW


DESCRIPTION SECTION
DESCRIPTION
Glipizide and metformin hydrochloride tablets, USP contain 2 oral antihyperglycemic drugs used in the management of type 2 diabetes, glipizide, USP and metformin hydrochloride, USP.
Glipizide, USP is an oral antihyperglycemic drug of the sulfonylurea class. The chemical name for glipizide, USP is 1-cyclohexyl-3-[[ p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea. Glipizide, USP is a whitish, odorless powder with a pK a of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide. The structural formula is represented below.
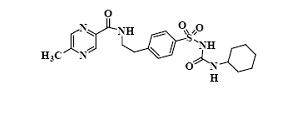
C 21H 27N 5O 4S M.W. 445.55
Metformin hydrochloride, USP is an oral antihyperglycemic drug used in the management of type 2 diabetes. Metformin hydrochloride, USP ( N, N-dimethylimidodicarbonimidic diamide monohydrochloride) is not chemically or pharmacologically related to sulfonylureas, thiazolidinediones, or α-glucosidase inhibitors. It is a white to off-white crystalline compound. Metformin hydrochloride, USP is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pK a of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride, USP is 6.68. The structural formula is as shown:
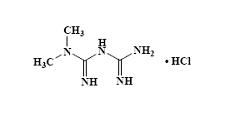
C 4H 12ClN 5 M.W. 165.63
Glipizide and metformin hydrochloride tablets, USP for oral administration contain 2.5 mg glipizide, USP with 250 mg metformin hydrochloride, USP, 2.5 mg glipizide, USP with 500 mg metformin hydrochloride, USP, and 5 mg glipizide, USP with 500 mg metformin hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: corn starch, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, povidone, talc, and titanium dioxide. Additionally, 2.5 mg/250 mg and 5 mg/500 mg tablets contain iron oxide black, iron oxide red, and iron oxide yellow. The tablets are film-coated, which provides color differentiation.
