Kit for the Preparation of Technetium Tc 99m Sulfur Colloid
These highlights do not include all the information needed to use KIT FOR THE PREPARATION OF TECHNETIUM Tc 99m SULFUR COLLOID INJECTION safely and effectively. See full prescribing information for KIT FOR THE PREPARATION OF TECHNETIUM Tc 99m SULFUR COLLOID INJECTION. KIT FOR THE PREPARATION OF TECHNETIUM Tc 99m SULFUR COLLOID INJECTION, for subcutaneous, intraperitoneal, intravenous and oral use. Initial U.S. Approval: 1978
f756e00e-0acd-4c5d-97f8-ed57f23a2393
HUMAN PRESCRIPTION DRUG LABEL
Dec 5, 2023
Jubilant DraxImage Inc., dba Jubilant Radiopharma
DUNS: 243604761
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Technetium Tc 99m Sulfur Colloid Kit
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
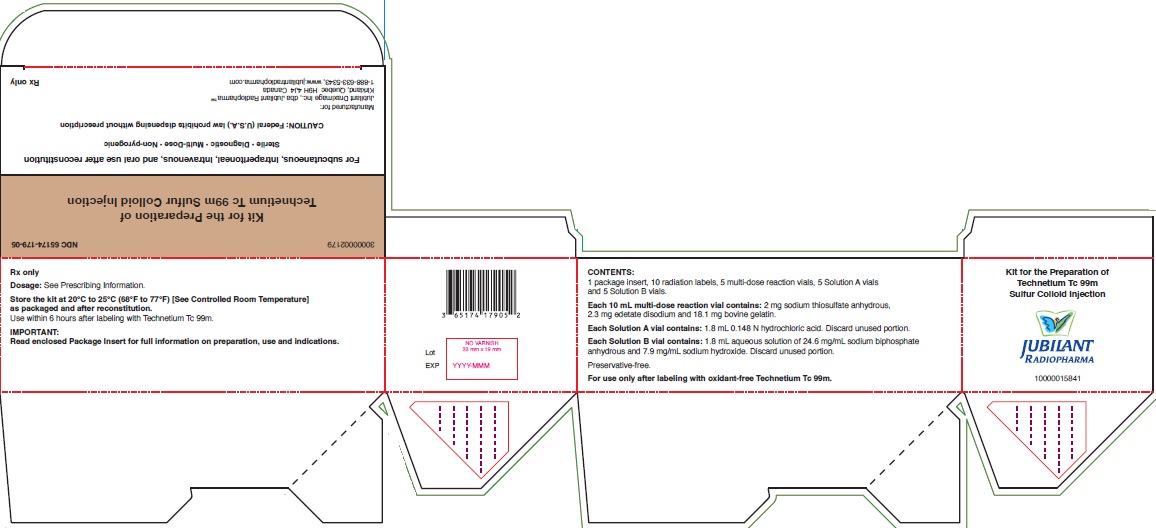
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - 5 VIAL BOX
NDC 65174-179-05
Kit for the Preparation of
Technetium Tc 99m Sulfur Colloid Injection
For subcutaneous, intraperitoneal, intravenous, and oral use after
reconstitution
Sterile • Diagnostic • Multi-Dose • Non-pyrogenic
CAUTION: Federal (U.S.A.) law prohibits dispensing without prescription
Manufactured for:
Jubilant DraxImage Inc., dba Jubilant Radiopharma™
Kirkland, Quebec H9H 4J4 Canada
1-888-633-5343, www.jubilantradiopharma.com
Rx only
Rx only
Dosage: See Prescribing Information.
Store the kit at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature] as packaged and after reconstitution.
Use within 6 hours after labeling with Technetium Tc 99m.
IMPORTANT:
Read enclosed Package Insert for full information on preparation, use and
indications.
CONTENTS:
1 package insert, 10 radiation labels, 5 multi-dose reaction vials, 5 Solution
A vials
and 5 Solution B vials.
Each 10 mL multi-dose reaction vial contains: 2 mg sodium thiosulfate
anhydrous,
2.3 mg edetate disodium and 18.1 mg bovine gelatin.
Each Solution A vial contains: 1.8 mL 0.148 N hydrochloric acid. Discard
unused portion.
Each Solution B vial contains: 1.8 mL aqueous solution of 24.6 mg/mL
sodium biphosphate
anhydrous and 7.9 mg/mL sodium hydroxide. Discard unused portion.
Preservative-free.
For use only after labeling with oxidant-free Technetium Tc 99m.
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Technetium Tc 99m Sulfur Colloid Injection is indicated:
In adults, to assist in the:
- Localization of lymph nodes draining a primary tumor in patients with breast cancer or malignant melanoma when used with a hand-held gamma counter.
- Evaluation of peritoneo-venous (LeVeen) shunt patency.
In adults and pediatric patients, for imaging:
- Areas of functioning reticuloendothelial cells in the liver, spleen and bone marrow.
- Studies of esophageal transit and gastroesophageal reflux, and detection of pulmonary aspiration of gastric contents.
Technetium Tc 99m Sulfur Colloid Injection is a radioactive diagnostic agent
indicated: (1)
In adults, to assist in the:
- Localization of lymph nodes draining a primary tumor in patients with breast cancer or malignant melanoma when used with a hand-held gamma counter.
- Evaluation of peritoneo-venous (LeVeen) shunt patency in adults.
In adults and pediatric patients for:
- Imaging areas of functioning reticuloendothelial cells in the liver, spleen and bone marrow.
- Studies of esophageal transit and gastroesophageal reflux, and detection of pulmonary aspiration of gastric contents.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None
None (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
Anaphylactic reactions with bronchospasm, hypotension, urticaria and rare fatalities have occurred following intravenously administered Technetium Tc 99m Sulfur Colloid Injection. Have emergency resuscitation equipment and personnel immediately available.
5.2 Radiation Risks
Radiation-emitting products, including Technetium Tc 99m Sulfur Colloid Injection, may increase the risk for cancer, especially in pediatric patients. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker. [see Dosage and Administration (2.3)]
5.3 Altered Distribution, Accumulation of Tracer in the Lungs
Technetium Tc 99m Sulfur Colloid Injection is physically unstable, and the particles will settle with time or with exposure to polyvalent cations. These larger particles are likely to be trapped by the pulmonary capillary bed following intravenous injection and result in non-uniform distribution of radioactivity. Agitate the vial adequately before administration of sulfur colloid to avoid particle aggregation and non-uniform distribution of radioactivity. Discard unused drug after 6 hours from the time of formulation. [see Dosage and Administration (2.2)]
Anaphylactic reactions including rare fatalities have occurred following intravenously administered Technetium Tc 99m Sulfur Colloid. Have resuscitation equipment and personnel immediately available. (5.1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The most frequently reported adverse reactions, across all categories of use and routes of administration, include rash, allergic reaction, urticaria, anaphylaxis/anaphylactic shock, and hypotension. Less frequently reported adverse reactions are fatal cardiopulmonary arrest, seizures, dyspnea, bronchospasm, abdominal pain, flushing, nausea, vomiting, itching, fever, chills, perspiration, numbness, and dizziness. Local injection site reactions, including burning, blanching, erythema, sclerosis, swelling, eschar, and scarring, have also been reported.
The most frequently reported adverse reactions include rash, urticaria, anaphylactic shock, and hypotension. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Jubilant DraxImage Inc., dba Jubilant RadiopharmaTM at 1-888-633-5343 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Specific drug-drug interactions have not been studied.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied in a package that contains 5 kits. All components of a kit are sterile and non-pyrogenic. Each kit contains three vials: one 10 mL Multi-Dose Reaction Vial, a Solution A vial and a Solution B vial. The vials contain the sterile non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Sulfur Colloid Injection. Each 10 mL Multi-Dose Reaction Vial contains, in lyophilized form, 2 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg bovine gelatin; each Solution A vial contains 1.8 mL 0.148 N hydrochloric acid solution and each Solution B vial contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. Included in each 5-kit package are one package insert and 10 radiation labels.
The Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied as a package that contains 5 kits. Each kit contains three vials: one 10 mL Multi-Dose Reaction Vial, a Solution A vial and a Solution B vial. The vials contain the sterile non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Sulfur Colloid Injection. (3)
OVERDOSAGE SECTION
10 OVERDOSAGE
The clinical consequences of overdosing with Technetium Tc 99m Sulfur Colloid Injection are not known.
REFERENCES SECTION
15 REFERENCES
• Bergqvist L, Strand S-E, Persson B, et al. Dosimetry in Lymphoscintigraphy
of Tc 99m Antimony Sulfide Colloid, J Nucl Med., 23: 698-705, 1982.
• Summary of Current Radiation Dose Estimates to Humans with Various Liver
Conditions from 99m Tc-Sulfur Colloid, MIRD Dose Estimate Report No 3, J Nucl
Med., 16: 108A-108B, 1975.
• Henrichs et al. Estimation of age-dependent internal dose from
radiopharmaceuticals, Phys. Med. Biol., 27: 775-784, 1982.
• Kocher DC: Radioactive decay data tables. DOE/TIC-11026: 108, 1981.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied in a package that contains 5 kits (NDC 65174-179-05). All kit components are sterile and non-pyrogenic. Each 10 mL Multi-Dose Reaction Vial (NDC 65174-131-01) contains, in lyophilized form, 2 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg bovine gelatin; each Solution A vial (NDC 65174-046-01) contains 1.8 mL 0.148 N hydrochloric acid solution and each Solution B vial (NDC 65174-047-01) contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. Included in each 5-kit package are one package insert and 10 radiation labels.
Store the kit at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature] as packaged and after reconstitution.
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection must comport with the product labeling and is approved for distribution to facilities and persons licensed by the U.S. Nuclear Regulatory Commission or under an equivalent license issued by an Agreement State.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Inform patients they may experience a burning sensation at the injection site.
Inform lactating woman to pump and discard breast milk for 24 hours after administration of Technetium Tc 99m Sulfur Colloid Injection and minimize close contact with infants for 6 hours after receiving a Technetium Tc 99m Sulfur Colloid Injection (8.2).
Manufactured for:

Jubilant DraxImage Inc., dba Jubilant RadiopharmaTM
16 751 TransCanada Highway
Kirkland, Quebec H9H 4J4 Canada
1-888-633-5343
www.jubilantradiopharma.com
Revised: December 2020
Art Rev.: 1.0
50000003096
Jubilant RadiopharmaTM is a trademark used under license by Jubilant DraxImage Inc.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with Technetium Tc 99m Sulfur Colloid Injection use in
pregnant women have not identified a drug-associated risk of major birth
defects, miscarriage or adverse maternal or fetal outcomes; technetium Tc 99m
crosses the placenta [see Data] . Animal reproduction studies have not been
conducted with Technetium Tc 99m Sulfur Colloid Injection. All
radiopharmaceuticals, including Technetium Tc 99m Sulfur Colloid Injection,
have a potential to cause fetal harm depending on the stage of fetal
development and the magnitude of the radiopharmaceutical dose. If considering
Technetium Tc 99m Sulfur Colloid Injection administration to a pregnant woman,
inform the patient about the potential for adverse pregnancy outcomes based on
the radiation dose from the drug and the gestational timing of exposure.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Among 14 infants born to pregnant patients exposed to Technetium Tc 99m Sulfur
Colloid Injection for lymph node localization, no birth defects were reported
following drug exposure.
8.2 Lactation
Risk Summary
Technetium Tc 99m is excreted in human milk during lactation. Exposure of
Technetium Tc 99m Sulfur Colloid Injection to a breastfed infant can be
minimized by temporary discontinuation of breastfeeding [see Clinical Considerations] . The developmental and health benefits of breastfeeding
should be considered along with the mother’s clinical need for Technetium Tc
99m Sulfur Colloid Injection and any potential adverse effects on the
breastfed child from Technetium Tc 99m Sulfur Colloid Injection or from the
underlying maternal condition.
Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating
woman to pump and discard breast milk for 24 hours after administration of
Technetium Tc 99m Sulfur Colloid Injection. Following higher dose procedures
[greater than 370 MBq (10 mCi)], patients should minimize close contact with
infants for 6 hours after receiving a Technetium Tc 99m Sulfur Colloid
Injection.
8.4 Pediatric Use
The safety and efficacy of Technetium Tc 99m Sulfur Colloid kit in pediatric patients has been shown for the following indications: liver, spleen, and bone marrow imaging, and gastroesophageal and pulmonary aspiration studies.
8.5 Geriatric Use
Clinical studies of Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Lactation: Advise a lactating woman to pump and discard breast milk for 24 hours and minimize close contact with infants for 6 hours after receiving a Technetium Tc 99m Sulfur Colloid Injection. (8.2)
DESCRIPTION SECTION
11 DESCRIPTION
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection contains a Multi-Dose Reaction Vial, a Solution A vial and a Solution B vial which contain the sterile non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Sulfur Colloid Injection for diagnostic use by subcutaneous, intraperitoneal, or intravenous injection or by oral administration.
Each 10 mL Multi-Dose Reaction Vial contains, in lyophilized form 2 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg bovine gelatin; a Solution A vial contains 1.8 mL of 0.148 N hydrochloric acid solution and a Solution B vial contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide.
When a solution of sterile and non-pyrogenic Sodium Pertechnetate Tc 99m Injection in isotonic saline is mixed with these components, following the instructions provided with the kit, Technetium Tc 99m Sulfur Colloid Injection is formed. The product is intended for subcutaneous, intraperitoneal, or intravenous injection or for oral administration. The precise structure of Technetium Tc 99m Sulfur Colloid Injection is not known at this time.
11.1 Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours4. The principal photon that is useful for detection and imaging studies is listed in Table 7.
Table 7. Principal Radiation Emission Data4|
** Radiation** |
** Mean Percent Per Disintegration** |
** Mean Energy (keV)** |
|
Gamma-2 |
89.07 |
140.5 |
4Kocher DC: Radioactive decay data tables. DOE/TIC-11026: 108, 1981.
11.2 External Radiation
The specific gamma ray constant for Tc 99m is 0.78 R/millicurie-hr at 1cm. The first half-value layer is 0.017 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 8. For example, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
Table 8. Radiation Attenuation by Lead Shielding|
** Shield Thickness (Pb) cm** |
** Coefficient of Attenuation** |
|
0.017 |
0.5 |
|
0.08 |
10-1 |
|
0.16 |
10-2 |
|
0.25 |
10-3 |
|
0.33 |
10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 9.
Table 9. Physical Decay Chart: Tc 99m, half-life 6.02 hours
| |||
|
Hours |
Fraction Remaining |
Hours |
Fraction Remaining |
|
0* |
1.000 |
6 |
0.501 |
|
1 |
0.891 |
7 |
0.447 |
|
2 |
0.794 |
8 |
0.398 |
|
3 |
0.708 |
9 |
0.355 |
|
4 |
0.631 |
10 |
0.316 |
|
5 |
0.562 |
11 |
0.282 |
|
|
|
12 |
0.251 |
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Technetium Tc 99m decays by isomeric transition, emitting a photon that can be detected for imaging purposes. [see Description (11.1)]
Following subcutaneous injection, Technetium Tc 99m Sulfur Colloid enters the lymphatic capillaries and is transported with lymph to lymph nodes. However, when there is massive nodal metastatic involvement, the normal transport to lymph nodes is lost because few normal cells remain in the node. [see Dosage and Administration (2.4)]
Following intraperitoneal injection, Technetium Tc 99m Sulfur Colloid mixes with the peritoneal fluid; rate of clearance from the cavity allows assessment of the patency of the shunt. Clearance varies from insignificant, which may occur with complete shunt blockage, to very rapid clearance with subsequent transfer into the systemic circulation when the shunt is patent.
Following intravenous injection, Technetium Tc 99m Sulfur Colloid is taken up by the reticuloendothelial system (RES), allowing RES rich structures to be imaged.
With oral administration, Technetium Tc 99m Sulfur Colloid is not absorbed accounting for its function in esophageal transit studies, gastroesophageal reflux scintigraphy, and for the detection of pulmonary aspiration of gastric contents.
12.3 Pharmacokinetics
Following intravenous administration, Technetium Tc 99m Sulfur Colloid Injection is rapidly cleared from the blood by the reticuloendothelial system with a nominal half-life of approximately 2 1/2 minutes. Uptake of the radioactive colloid by organs of the RES is dependent upon both their relative blood flow rates and the functional capacity of the phagocytic cells. In the average patient 80 to 90% of the injected collodial particles are phagocytized by the Kupffer cells of the liver, 5 to 10% by the spleen and the balance by the bone marrow.
Following oral ingestion, Technetium Tc 99m Sulfur Colloid is distributed primarily through the gastrointestinal tract with elimination primarily through the feces.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies to evaluate the carcinogenicity, mutagenesis, or reproductive toxicity potentials of Technetium Tc 99m Sulfur Colloid have not been conducted.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Tracer Localization to Lymph Nodes in Breast Cancer
A systematic review of 43 publications examined procedures that used the injection of Technetium Tc 99m Sulfur Colloid Injection and a blue dye (tracers) to assist surgeons in the localization of lymph nodes among patients with a primary breast cancer lesion. From these publications, 15 studies were identified for inclusion within a meta-analysis, based upon the following criteria: prospective design, minimum number of 50 lymph node localization procedures, and paired outcome data available for both Technetium Tc 99m Sulfur Colloid Injection and blue dye. Within these studies, the number of procedures ranged from 62 to 6,197; in general one procedure involved a single patient but in some uncommon situations, one patient underwent more than one procedure. The patients received subcutaneous Technetium Tc 99m Sulfur Colloid Injection doses ranging between 0.1 and 2 mCi. The mean age of patients ranged from 52 to 60 years, and almost all were female. Lymph nodes that contained radioactivity were generally localized based upon increased counts, in comparison to a background threshold (e.g., nodes containing a minimum of radioactive counts 3 times higher than background or containing at least 10 fold higher counts than contiguous nodes). Radioactivity was measured using a handheld gamma counter.
Table 10 shows the tracer localization rates where the tracer localization rate (%) is defined as the percentage of procedures which had at least one lymph node containing the specific tracer. Random effect meta-analytic measures were used for estimating various rates of tracer localization by procedure along with the respective confidence intervals. The random effect meta-analytical methods take into account the sample size of each study as well as within and between study variability. In general, most procedures involved the resection of lymph nodes in which a tracer had localized to at least one node. However, in some procedures (estimated at approximately 3.4%) neither tracer was localized to a resected lymph node. The reports were insufficient to establish the basis for failed tracer localization. [see Dosage and Administration (2.4)]
Table 10. Tracer Localization by Procedure - Breast Cancer*|
** Number of Clinical Studies** |
** Number of Procedures** |
** BD Present (%)** |
** SCI Present (%)** |
** Only BD Present (%)** |
Only SCI |
Neither SCI nor BD Present (%) |
|
15 |
9,213 |
85.1 |
94.1 |
3.8 |
12.1 |
3.4 |
|
95% Confidence Intervals** |
81.4, 88.2 |
91.4, 96.0 |
2.8, 5.2 |
9.9, 15.0 |
2.1, 5.4 |
BD = blue dye, SCI = Technetium Tc 99m Sulfur Colloid Injection
- Percentage of procedures in which at least one lymph node contained the specific tracer; the percents do not add to 100% due to rounding.
** 95% Confidence Intervals are based on meta-analysis and represent the spread in the individual estimates.
In some of the publications, different methods of Technetium Tc 99m Sulfur Colloid Injection administration were compared: intradermal (ID), subareolar (SA) and intraparenchymal (IP) methods. Generally, more favorable results were seen using the ID and SA routes, with less favorable results reported when surgeons used the IP method.
14.2 Tracer Localization to Lymph Nodes in Malignant Melanoma
A systematic review of eight publications examined the use of Technetium Tc 99m Sulfur Colloid and a blue dye (tracers) to assist surgeons in the localization of lymph nodes among patients with malignant melanoma. A meta- analysis was performed using data from the studies that reported the resected lymph node content of Technetium Tc 99m Sulfur Colloid Injection and blue dye. Four of the eight publications met this criterion and were included in the meta-analysis. Within these four studies, the number of reported patients ranged from 12 to 94. The patients received subcutaneous Technetium Tc 99m Sulfur Colloid Injection doses ranging between 0.25 to 2 mCi. The patients were aged 15 to 89 years and most (53 to 70%) were male.
Lymph nodes that contained radioactivity were generally localized based upon increased counts, in comparison to a background threshold (e.g., nodes containing a minimum of radioactive counts 3 times higher than background). Radioactivity was measured using a handheld gamma counter.
Table 11 shows the tracer localization rates where the tracer localization rate (%) is defined as the percentage of patients who had at least one lymph node containing the specific tracer. Random effect meta-analytic measures were used for estimating the various rates of tracer localization by patient along with the respective confidence intervals. The random effect meta-analytical methods take into account the sample size of each study as well as within and between study variability. In general, most patients had resected lymph nodes that contained at least one of the tracers. However, in some patients (estimated at approximately 1.6%) neither tracer was localized to a resected lymph node. The reports were insufficient to establish the basis for failed tracer localization. [see Dosage and Administration (2.4)]
Table 11. Tracer Localization by Patient Malignant Melanoma*|
** Number of Clinical Studies** |
** Number of Patients** |
** BD Present (%)** |
** SCI Present (%)** |
** Only BD Present (%)** |
** Only SCI Present (%)** |
** Neither SCI nor BD Present (%)** |
|
4 |
249 |
83.6 |
96.4 |
3.2 |
15.5 |
1.6 |
|
95% Confidence Intervals** |
73.4, 90.4 |
92.0, 98.5 |
1.4, 6.9 |
9.6, 24.1 |
0.4, 6.5 |
BD = blue dye, SCI = Technetium Tc 99m Sulfur Colloid Injection
- Percentage of patients in which at least one lymph node contained the specific tracer; the percents do not add to 100% due to rounding.
** 95% Confidence Intervals are based on meta-analysis and represent the spread in the individual estimates.
