NATPARA (parathyroid hormone)
These highlights do not include all the information needed to use NATPARA safely and effectively. See full prescribing information for NATPARA. NATPARA (parathyroid hormone) for injection, for subcutaneous use Initial U.S. Approval: 2015
d11fba31-0a6c-11e3-8ffd-0800200c9a66
HUMAN PRESCRIPTION DRUG LABEL
May 22, 2023
Takeda Pharmaceuticals America, Inc.
DUNS: 039997266
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
PARATHYROID HORMONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
PARATHYROID HORMONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
PARATHYROID HORMONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
PARATHYROID HORMONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
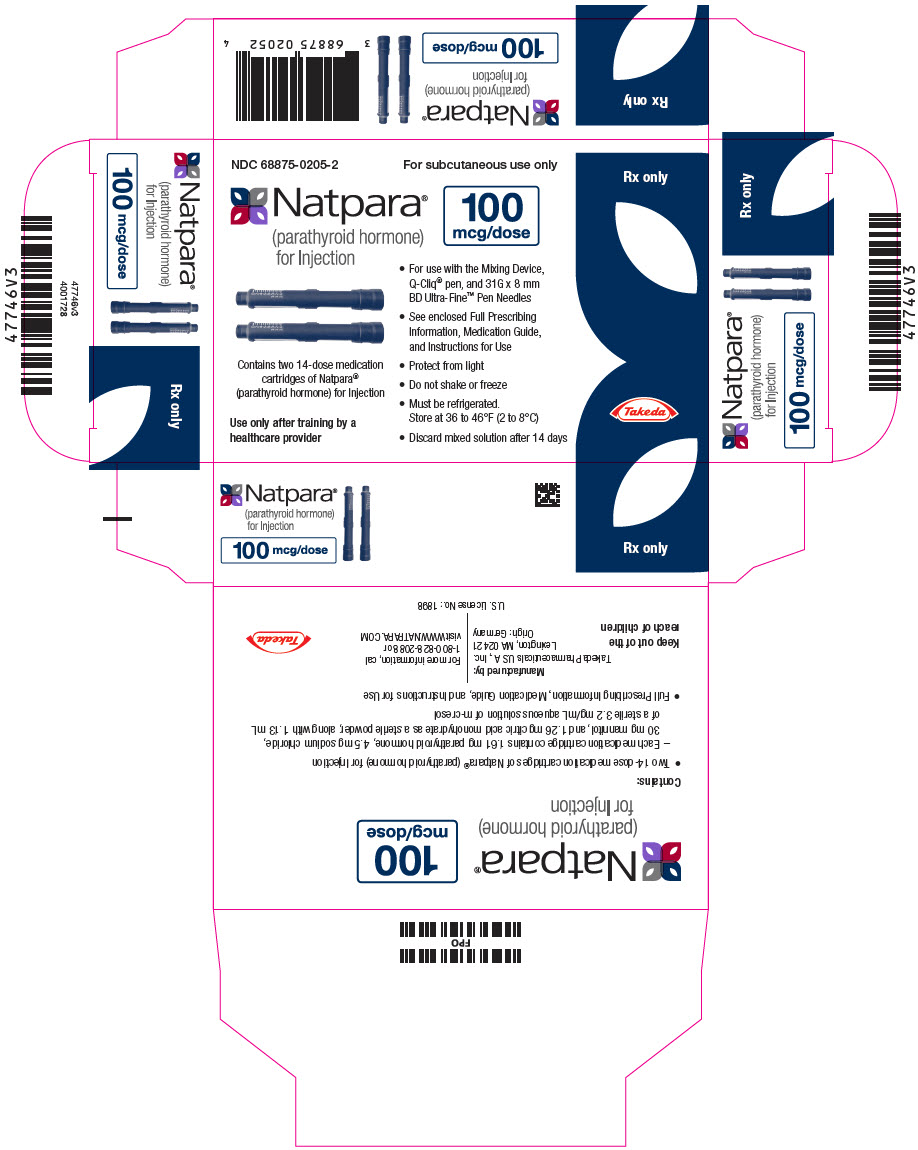
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mcg Cartridge Carton
NDC 68875-0205-2
For subcutaneous use only
Natpara®
(parathyroid hormone)
for Injection
100
mcg/dose
Contains two 14-dose medication
cartridges of Natpara®
(parathyroid hormone) for Injection
Use only after training by a
healthcare provider
-
For use with the Mixing Device,
Q-Cliq® pen, and 31G x 8 mm
BD Ultra-Fine™ Pen Needles -
See enclosed Full Prescribing
Information, Medication Guide,
and Instructions for Use -
Protect from light
-
Do not shake or freeze
-
Must be refrigerated.
Store at 36 to 46°F (2 to 8°C) -
Discard mixed solution after 14 days
Rx only
Takeda
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Potential Risk of Osteosarcoma
In male and female rats, parathyroid hormone caused an increase in the incidence of osteosarcoma (a malignant bone tumor). The occurrence of osteosarcoma was observed to be dependent on parathyroid hormone dose and treatment duration. This effect was observed at parathyroid hormone exposure levels ranging from 3 to 71 times the exposure levels for humans receiving a 100 mcg dose of NATPARA. These data could not exclude a risk to humans [see Nonclinical Toxicology (13.1)].
Because of a potential risk of osteosarcoma, use NATPARA only in patients who cannot be well-controlled on calcium supplements and active forms of vitamin D alone and for whom the potential benefits are considered to outweigh this potential risk [see Limitations of Use (1)].
To further mitigate the potential risk of osteosarcoma, avoid use of NATPARA in patients who are at increased risk for osteosarcoma, such as patients with Paget's disease of bone or unexplained elevations of alkaline phosphatase, pediatric and young adult patients with open epiphyses, patients with hereditary disorders predisposing to osteosarcoma, or patients with a prior history of external beam or implant radiation therapy involving the skeleton. Instruct patients to promptly report clinical symptoms (e.g., persistent localized pain) and signs (e.g., soft tissue mass tender to palpation) that could be consistent with osteosarcoma.
NATPARA is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 NATPARA REMS Program
Because of the potential risk of osteosarcoma associated with NATPARA therapy, NATPARA is available only through a restricted REMS program called the NATPARA REMS Program. Under the NATPARA REMS Program, only certified healthcare providers can prescribe and only certified pharmacies can dispense NATPARA. Further information is available at www.NATPARAREMS.com or by telephone at 1-855-NATPARA (1-855-628-7272).
5.3 Hypercalcemia
Severe hypercalcemia has been reported with NATPARA. In the pivotal trial, 3 patients randomized to NATPARA required administration of IV fluids to correct hypercalcemia during treatment with NATPARA. The risk is highest when starting or increasing the dose of NATPARA, but can occur at any time. Monitor serum calcium and patients for signs and symptoms of hypercalcemia. Treat hypercalcemia per standard practice and consider holding and/or lowering the dose of NATPARA if severe hypercalcemia occurs [see Dosage and Administration (2), Adverse Reactions (6.1)].
5.4 Hypocalcemia
Severe hypocalcemia has been reported in patients taking NATPARA, including cases of hypocalcemia that resulted in seizures. The risk is highest when NATPARA is withheld, missed or abruptly discontinued, but can occur at any time. Monitor serum calcium and patients for signs and symptoms of hypocalcemia. Resume treatment with, or increase the dose of, an active form of vitamin D or calcium supplements or both if indicated in patients interrupting or discontinuing NATPARA to prevent severe hypocalcemia [see Dosage and Administration (2.6), Adverse Reactions (6.1)].
5.5 Risk of Digoxin Toxicity with Concomitant Use of Digitalis Compounds
The inotropic effects of digoxin are affected by serum calcium levels. Hypercalcemia of any cause may predispose to digoxin toxicity. In patients using NATPARA concomitantly with digitalis compounds, monitor serum calcium and digoxin levels and patients for signs and symptoms of digitalis toxicity. Adjustment of digoxin and/or NATPARA may be needed. No drug-drug interaction study has been conducted with digoxin and NATPARA [see Drug Interactions (7), Adverse Reactions (6.1)].
5.6 Hypersensitivity
There have been reports of hypersensitivity reactions in patients taking NATPARA. Reactions included anaphylaxis, dyspnea, angioedema, urticaria, and rash. If signs or symptoms of a serious hypersensitivity reaction occur, discontinue treatment with NATPARA, treat hypersensitivity reaction according to the standard of care, and monitor until signs and symptoms resolve [see Contraindications (4), Adverse Reactions (6.3)]. Monitor for hypocalcemia if NATPARA is discontinued [see Dosage and Administration (2.6)].
- Potential Risk of Osteosarcoma: Prescribe NATPARA only to patients who cannot be well-controlled on calcium and active vitamin D. Avoid use of NATPARA in patients who are at increased risk for osteosarcoma. (5.1)
- Severe Hypercalcemia: Monitor serum calcium when starting or adjusting NATPARA dose and when making changes to co-administered drugs known to raise serum calcium. (2.4, 5.3, 6.1)
- Severe Hypocalcemia: Can occur with interruption or discontinuation of NATPARA treatment. Monitor serum calcium and replace calcium and vitamin D. (2.4, 5.4, 6.1)
- Digoxin Toxicity: Hypercalcemia increases the risk of digoxin toxicity. In patients using NATPARA concomitantly with digoxin, monitor serum calcium more frequently and increase monitoring when initiating or adjusting NATPARA dose. (5.5)
- Hypersensitivity: Hypersensitivity reactions (anaphylaxis, dyspnea, angioedema, urticaria, rash) have been reported. If signs or symptoms of a serious hypersensitivity reaction occur, discontinue treatment with NATPARA and treat patient according to the standard of care (2.6, 4, 5.6, 6.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail in other sections of the label:
- Osteosarcoma [see Boxed Warning, Warnings and Precautions (5.1)]
- Hypercalcemia [see Warnings and Precautions (5.3)]
- Hypocalcemia [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Contraindications (4), Warnings and Precautions (5.6)]
6.1 Adverse Reactions in Clinical Trials for Hypoparathyroidism
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
NATPARA was studied in a placebo-controlled trial [see Clinical Studies (14)].
The data described in Table 2 below reflect exposure to NATPARA in 84 patients, including 78 exposed for 24 weeks. The mean age of the trial population was 47 years and ranged from 19 to 74 years old. Seventy-nine percent (79%) were females. Ninety-six percent (96%) were Caucasian, 0.8% were Black, and 1.6% were Asian. Patients had had hypoparathyroidism for on average 15 years and hypoparathyroidism was caused by post-surgical complications in 71% of cases, idiopathic hypoparathyroidism in 25% of cases, DiGeorge Syndrome in 3% of cases, and auto-immune hypoparathyroidism in 1% of cases. Prior to trial enrollment, participants were receiving a median (interquartile range) daily oral calcium dose of 2000 (1250, 3000) mg and a median daily oral active vitamin D dose equivalent to 0.75 (0.5, 1) mcg of calcitriol. The mean eGFR at baseline was 97.4 mL/min/1.73 m2 and 45%, 10% and 0% had mild, moderate and severe renal impairment, respectively, at baseline. During the trial, most patients received 100 mcg and the dose range was 50 to 100 mcg administered subcutaneously once daily in the thigh.
Table 2 lists common adverse reactions associated with NATPARA use in the clinical trial. Common adverse reactions were reactions that occurred in ≥5% of subjects and occurred more commonly on NATPARA than on placebo.
Table 2: Common Adverse Reactions associated with NATPARA use in Subjects with Hypoparathyroidism|
Adverse Reaction |
Placebo |
NATPARA |
|---|---|---|
| ||
|
Paresthesia |
25 |
31 |
|
Hypocalcemia* |
23 |
27 |
|
Headache |
23 |
25 |
|
Hypercalcemia* |
3 |
19 |
|
Nausea |
18 |
18 |
|
Hypoesthesia |
10 |
14 |
|
Diarrhea |
3 |
12 |
|
Vomiting |
0 |
12 |
|
Arthralgia |
10 |
11 |
|
Hypercalciuria* |
8 |
11 |
|
Pain in extremity |
8 |
10 |
|
Upper respiratory tract infection |
5 |
8 |
|
Abdominal pain upper |
3 |
7 |
|
Sinusitis |
5 |
7 |
|
Blood 25-hydroxycholecalciferol decreased |
3 |
6 |
|
Hypertension |
5 |
6 |
|
Hypoesthesia facial |
3 |
6 |
|
Neck pain |
3 |
6 |
Hypercalcemia
In the overall pivotal trial, a greater proportion of patients on NATPARA had albumin-corrected serum calcium above the normal range (8.4 to 10.6 mg/dL). During the entire trial duration 3 patients on NATPARA and 1 patient on placebo had a calcium level above 12 mg/dL. Table 3 displays the number of subjects who had albumin-corrected serum calcium levels above the normal range (8.4 to 10.6 mg/dL) by study treatment period in the placebo-controlled study based on routine monitoring at each trial visit. More patients randomized to NATPARA had hypercalcemia in both phases of the study (note: all trial participants underwent a 50% reduction in active vitamin D dose at randomization).
Table 3: Proportion of Subjects with Albumin-Corrected Serum Calcium Greater Than Upper Limit of Normal (10.6 mg/dL) During the Treatment Period|
Titration Period |
Maintenance Period | |||
|---|---|---|---|---|
|
Albumin-corrected serum calcium |
Placebo |
NATPARA |
Placebo |
NATPARA |
| ||||
|
0% |
30% |
0% |
10% |
|
0% |
2% |
3% |
0% |
Hypocalcemia
Table 4 displays the number of subjects who had albumin-corrected serum calcium levels below 8.4 mg/dL by treatment period in the placebo-controlled study based on routine monitoring at each trial visit. More patients randomized to placebo had hypocalcemia of less than 7 mg/dL in the titration phase (note: all trial participants underwent a 50% reduction in active vitamin D dose at randomization). More patients randomized to NATPARA had hypocalcemia of less than 7 mg/dL in the dose maintenance phase.
Table 4: Proportion of Subjects with Albumin-Corrected Serum Calcium Below the Lower Limit of Normal (8.4 mg/dL) During the Treatment Period|
Titration Period |
Maintenance Period | |||
|---|---|---|---|---|
|
Albumin-corrected serum calcium |
Placebo |
NATPARA |
Placebo |
NATPARA |
|
≥7 to <8.4 mg/dL |
98% |
79% |
75% |
71% |
|
<7 mg/dL |
18% |
6% |
0% |
12% |
The risk of hypocalcemia increases when NATPARA is withdrawn. At the end of the trial, NATPARA and placebo were withdrawn, calcium and active vitamin D were returned to baseline doses and subjects were followed for 4 weeks. During this withdrawal phase, more patients previously randomized to NATPARA experienced an albumin-corrected serum calcium value of less than 7 mg/dL (5.0% versus 17% for previous treatment with placebo and NATPARA, respectively). Twenty subjects (24%) previously randomized to NATPARA experienced adverse reactions of hypocalcemia in the post-treatment phase compared to three subjects (8%) previously randomized to placebo. Five subjects previously randomized to NATPARA with albumin-corrected serum calcium below 7 mg/dL required treatment with IV calcium gluconate to correct hypocalcemia.
Hypercalciuria
Treatment with NATPARA did not lower 24-hour urinary calcium excretion in the placebo-controlled trial. The proportion of subjects with hypercalciuria (defined as urine calcium levels of >300 mg/24 hours) was similar at baseline and trial end in the NATPARA and placebo groups. The median (IQR) 24-hour Urine Calcium at trial end was similar between NATPARA [231 (168-351) mg/24 hours], and placebo [232 (139-342) mg/24 hours]. At trial end, serum calcium values between NATPARA and placebo were also similar. Risk of hypercalciuria throughout the trial was related to serum calcium levels. To minimize the risk of hypercalciuria, NATPARA should be dosed to a target albumin-corrected total serum calcium within the lower half of the normal range (i.e., between 8 and 9 mg/dL) [see Dosage and Administration (2.1)].
6.2 Immunogenicity
NATPARA may trigger the development of antibodies. In the placebo-controlled study in adults with hypoparathyroidism, the incidence of anti-PTH antibodies was 8.6% (3/35) and 5.9% (1/17) in subjects who received subcutaneous administration of 50 to 100 mcg NATPARA or placebo once daily for 24 weeks, respectively.
Across all clinical studies in subjects with hypoparathyroidism following treatment with NATPARA for up to 2.6 years, the immunogenicity incidence rate was 16.1% (14/87). These 14 subjects had low titer anti-PTH antibodies and, of these, 3 subjects subsequently became antibody negative. One of these subjects had antibodies with neutralizing activity; this subject maintained a clinical response with no evidence of immune-related adverse reactions. Anti-PTH antibodies did not appear to affect efficacy or safety during the clinical trials but their longer-term impact is unknown.
Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying diseases. For these reasons, comparison of the incidence of antibodies to NATPARA with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NATPARA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions (e.g., anaphylaxis, dyspnea, angioedema, urticaria, and rash).
- Seizures due to hypocalcemia
- The most common adverse reactions associated with NATPARA and occurring in greater than 10% of individuals were: paresthesia, hypocalcemia, headache, hypercalcemia, nausea, hypoesthesia, diarrhea, vomiting, arthralgia, hypercalciuria and pain in extremity (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-800-828-2088 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Alendronate
Co-administration of alendronate and NATPARA leads to reduction in the calcium-sparing effect, which can interfere with the normalization of serum calcium. Concomitant use of NATPARA with alendronate is not recommended.
7.2 Digoxin
NATPARA causes transient increase in calcium and therefore, concomitant use of NATPARA and cardiac glycosides (e.g., digoxin) may predispose patients to digitalis toxicity if hypercalcemia develops. Digoxin efficacy is reduced if hypocalcemia is present. In patients using NATPARA concomitantly with digoxin, carefully monitor serum calcium and digoxin levels, and patients for signs and symptoms of digoxin toxicity. Adjustment of digoxin and/or NATPARA may be needed. No drug-drug interaction study has been conducted with digoxin and NATPARA.
- Digoxin: Monitor serum calcium more frequently when using NATPARA in patients receiving digoxin. (5.5, 7.2)
INSTRUCTIONS FOR USE SECTION
Instructions for Use
NATPARA® (nat-PAH-rah)
(parathyroid hormone)
for injection, for subcutaneous use
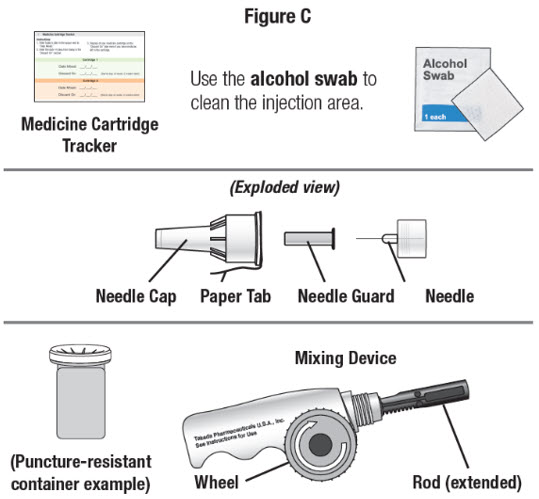
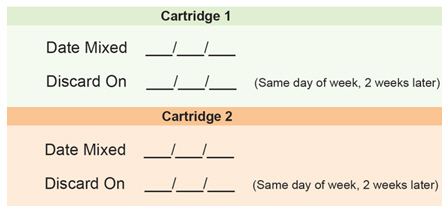
Medicine Cartridge Tracker
Instructions:
1. Enter today's date in the space next to "Date Mixed."
2. Enter the date 14 days from today in the "Discard On" section.
3. Dispose of your medicine cartridge on the "Discard On" date even if you have medicine left in the cartridge.
Read this Instructions for Use before you start using NATPARA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Before you use NATPARA for the first time, make sure your healthcare provider shows you the right way to use it.
Table of Contents
Getting to Know the Parts of Your Q-Cliq Pen and Your NATPARA Medicine
Preparing and Mixing Your NATPARA
Preparing Your Q-Cliq Pen
Giving Your Daily NATPARA
Frequently Asked Questions
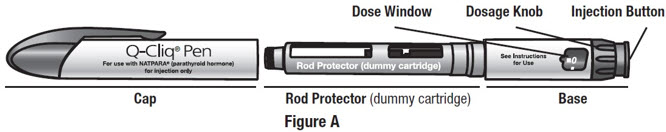
Getting to Know the Parts of Your Q-Cliq Pen and Your NATPARA Medicine
Your Q-Cliq pen can be re-used for up to 2 years.SeeFigure A.
The rod protector protects the rod during shipment from the factory. Throw away the rod protector when you are ready to use your Q-Cliq pen.
Your NATPARA Medicine Cartridge:
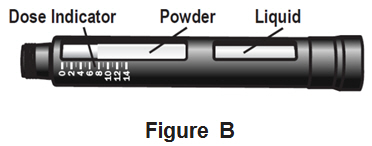
- Your NATPARA medicine cartridge contains medicine powder and a liquid to mix the powder in.SeeFigure B. You must mix the powder and the liquid in the medicine cartridge before using your Q-Cliq pen.
- Each medicine cartridge contains14 doses.
- The dose indicator shows you the number of doses left in the medicine cartridge.
|
Other supplies you will need to give your NATPARA. SeeFigure C. |
|
|
Preparing and Mixing Your NATPARA
- Mix your NATPARA1 time every14 days.
- If this is your first time using NATPARA by yourself, a trainer will guide you through how to mix your first NATPARA medicine cartridge and also your second medicine cartridge.
Step 1. Wash and dry your hands.
Step 2. Gather your supplies, including:
- your Q-Cliq pen
- your mixing device
- new NATPARA medicine cartridge from the refrigerator
- new disposable Pen Needle
- puncture-resistant sharps container
- pencil or pen
- Medicine Cartridge Tracker
Step 3. Look at theMedicine Cartridge Tracker in the front of your NATPARA booklet.
|
Step 4. Write the date in the space next to "Date Mixed."See Figure D. |
|
|
Step 5. Write the date14 days from the day you mixed NATPARA in the space next to "Discard on." This will be the same day of the week2 weeks later.SeeFigure E. |
|
|
*Do not use your NATPARA on or after the "Discard on" date even if there is medicine left in the cartridge.
| |
|
Step 6. Remove the paper tab from the needle cap.SeeFigure F. |
|
|
Step 7. While keeping the needle cap straight, screw it firmly onto the
cartridge in a clockwise direction. |
|
|
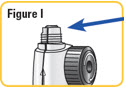
Step 8. Turn the wheel of the mixing device in a counterclockwise direction to lower the rod if it is not already lowered.SeeFigure H. |
|
|
|
|
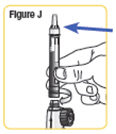
Step 9. Screw the cartridge onto your NATPARA mixing device in a clockwise direction. The Pen Needle must be attached.SeeFigure J. |
|
|
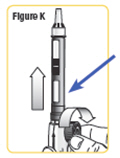
Step 10. With the needle cap pointing up, turn the wheel slowly in a clockwise direction until the stoppers no longer move.Make sure the wheel turns easily. SeeFigure K. |
|
|
Step 11. Make sure the stoppers look like this and stay together.See Figure L. |
|
|
Step 12. Mix the powder by slowly moving the cartridge back and forth
about10 times. |
|
Step 13. Set the mixing device down with the medicine cartridge attached. Wait until the powder is dissolved and the liquid is colorless, or5 minutes have passed. It is normal to see small particles in the liquid.
Step 14. If the liquid is colorless,go to Step 15. If the liquid is not colorless, call 1-800-828-2088 for help.
Preparing Your Q-Cliq Pen
You will prepare your Q-Cliq pen1 time every14 days
|
Step 15. Pick up your Q-Cliq pen and remove the cap. |
|
|
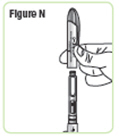
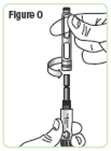
Step 16. Unscrew the rod protector or the empty medicine cartridge in a counterclockwise direction and throw it away in a puncture-resistant sharps container.SeeFigure O. |
|
|
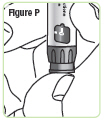
Step 17. Press the injection button. You should see the "0" line up with the notch in the dose window. If you do not see the "0" line up, press the injection button until it is lined up.SeeFigure P. |
|
|
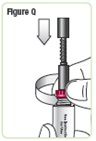
Step 18. Lower the rod. If the rod is extended, turn the dark red ring counterclockwise to lower it. Do not tighten the ring too much.See Figure Q. |
|
|
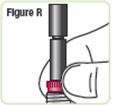
Step 19. Check the rod. It will have a small space when done the right way.SeeFigure R. |
|
|
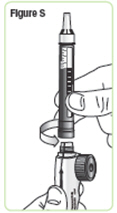
Step 20. Pick up the mixing device with the needle cap pointing up. Unscrew the cartridge from the mixing device in a counterclockwise direction and set the mixing device down.SeeFigure S. |
|
|
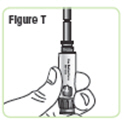
Step 21. Attach the medicine cartridge to the pen. Pick up the Q-Cliq pen base and hold it with the rod pointed upright.SeeFigure T. |
|
|
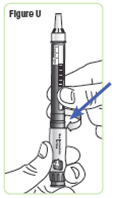
Step 22. With the needle cap pointing up, screw the medicine cartridge onto the Q-Cliq pen in a clockwise direction until there is no space between the medicine cartridge and the Q-Cliq pen.SeeFigure U. |
|
Priming your Q-Cliq pen.
|
Step 23. Turn the dosage knob until "GO" lines up with the notch in the dose window.SeeFigure V. |
|
|
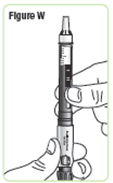
Step 24. Hold the Q-Cliq pen with the needle cap pointing up.See Figure W. |
|
|
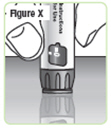
Step 25. Press the injection button on a flat surface, such as a table top, until the "0" lines up with the notch in the dose window.See Figure X. |
|
- It is normal for1 or2 drops of liquid to leak out during this step. *Do not remove the medicine cartridge from the Q-Cliq pen until the "Discard on" date or the medicine cartridge is empty.
- Prime your Q-Cliq pen only1 time for each new medicine cartridge.
Giving Your Daily NATPARA
- If you have just finished mixing your medicine and preparing your Q-Cliq pen and the Pen Needle is on,go to Step 33.
If you need help at any time, call 1-800-828-2088
Step 26. Wash and dry your hands.
Step 27. Gather your supplies, including:
- your Q-Cliq pen from the refrigerator
- new disposable Pen Needle
- puncture resistant sharps container
|
Step 28. Remove the cap from the Q-Cliq pen. The mixed medicine cartridge should be inside. |
|
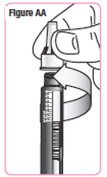
Step 29. Check the medicine inside the cartridge.SeeFigure Y.
Step 30. If the liquid is colorless, go toStep 31. It is normal to see small particles in the liquid. If the liquid is not colorless, call 1-800-828-2088 for help.
Attaching a new Pen Needle
|
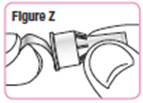
Step 31. Remove the paper tab from the needle cap.SeeFigure Z. |
|
|
Step 32. While keeping the needle cap straight, screw it firmly onto the
medicine cartridge in a clockwise direction. |
|
|
Step 33. Clean the area of your thigh where you will give NATPARA with an
alcohol swab. |
|
Make sure the needle cap is pointing downward at all times during Step 34 to Step 39.
|
Step 34. Hold the Q-Cliq pen with the needle cappointing down until after your injection.SeeFigure CC. |
|
|
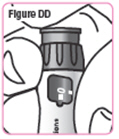
Step 35. Hold the Q-Cliq pen so you can see the dose window.See Figure DD. |
|
|
Step 36. Turn the dosage knob until "GO" lines up with the notch in the window.Do not turn the dosage knob past "GO."SeeFigure EE. |
|
*If the dosage knob is hard to turn, you may not have enough liquid left.
Check thedose indicator on the medicine cartridge to see if there are any
doses left or check the "Discard on" date on theMedicine Cartridge
Tracker to see if it has been more than14 days.
|
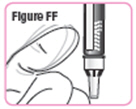
Step 37. Gently tap the medicine cartridge3 to 5 times.See Figure FF. |
|
Prepare the Pen Needle for giving the injection:
|
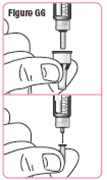
Step 38. Pull theneedle cap off and set it aside. Make sure youdo not unscrew the needle cap. Pull theneedle guard off and throw it away. See****Figure GG. |
|
|
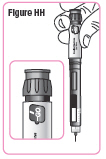
Step 39. Hold the Q-Cliq pen so you can see**"GO"** in the dose window with the Pen Needle pointing down.SeeFigure HH. |
|
Giving your NATPARA Injection
|
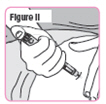
Step 40. Insert the Pen Needle fully into your thigh. Make sure you can see**"GO"** in the window.SeeFigure II. |
|
|
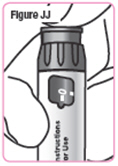
Step 41. Press the injection button until the**"0"** lines up with the notch in the dose window. You should see and feel the dosage knob turn back to "0." SeeFigure JJ. |
|
|
|
Step 42. Remove the Pen Needle from your skin by pulling it straight out.
- It is normal for1 or2 drops of liquid to leak out during this step.
- If you do not think you received your full dose, do not take another dose. Call your healthcare provider. You may need to take calcium and active vitamin D.
|
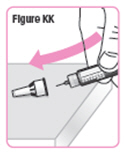
Step 43. Carefully put the large needle cap back on the Pen Needle by scooping the cap back on the needle using only1 hand.SeeFigure KK. |
|
|
Step 44. Unscrew the needle cap (with Pen Needle inside) in a counterclockwise direction while holding the medicine cartridge.See Figure LL. |
|
- Do not share your Q-Cliq pen or Pen Needles with anyone else. You may give an infection to them or get an infection from them.
After your injection:
Step 45. Discard your used needles and medicine cartridges
|
|
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 46. Put the cap back on your Q-Cliq pen
|
|
Step 47. Put your Q-Cliq pen in the refrigerator.
How should I store NATPARA?
*Unmixed NATPARA medicine cartridges: Refrigerate NATPARA between 36°F to 46°F (2°C to 8°C).Do not freeze. *Mixed NATPARA medicine cartridges: * Refrigerate between 36°F to 46°F (2°C to 8°C).Do not freeze. * You can use the Q-Cliq pen for up to14 days after mixing the medicine cartridge. * Throw away the mixed NATPARA medicine cartridges14 days after mixing the medicine cartridge.
- Store NATPARA away from heat and light. *Do not freeze or shake NATPARA.Do not use NATPARA if it was frozen or shaken.
Keep NATPARA and all medicines out of the reach of children.
Frequently Asked Questions
There are 4 ways to get your questions answered:
- Call your healthcare provider.
- Visit our Website at www.NATPARA.com.
- Call 1-800-828-2088.
- Find it in the following list of questions.
What does the dose window in the Q-Cliq pen tell me?
The dose window tells you if the Q-Cliq pen is ready for the injection:
- "0" means not ready
- "GO" means ready
- The dose window does not count the number of doses left or given
Why does the dose indicator scale on the medicine cartridge show "14" after I have given an injection using a newly mixed NATPARA medicine cartridge?
You might have forgotten to prime the Q-Cliq pen. SeeStep 23 in the "Priming your Q-Cliq pen." section of the Instructions for Use. Call 1-800-828-2088 for help.
What if the dosage knob is hard to turn to "GO"?
- Do not use force if the knob will not easily turn to "GO." You may have used your last dose.
- Check the dose indicator on the medicine cartridge to see if there are any doses left, or check the "Discard on" date on theMedicine Cartridge Tracker to see if it has been more than14 days.
- If the cartridge contains at least1 dose, call 1-800-828-2088 for help.
What should I do if the NATPARA medicine cartridge is frozen, whether or not it is attached to the Q-Cliq pen?
Throw away the frozen medicine cartridge and mix a new medicine cartridge.
Why do I not throw away the medicine cartridge on day 14 after giving the last injection?
A medicine cartridge or the "dummy" rod protector is needed to put the cap on the Q-Cliq pen. After injecting yourself on day14, leave the current medicine cartridge on the Q-Cliq pen, put the pen cap back on, and store the pen in the refrigerator. The next day, which will be day15, throw away the old medicine cartridge and mix a new one.
What if the newly mixed NATPARA medicine cartridge is hard to screw onto the Q-Cliq pen?
- The rod in the Q-Cliq pen may be extended.
- Remove the cartridge and make sure that the rod is fully lowered. If it is not fully lowered, turn the dark red ring to lower it until the ring stops.Do not tighten it too much. Reattach the cartridge and see if it is easier to attach.
- If it is still too hard to screw onto the Q-Cliq pen, check to see if the stoppers in the cartridge window are together.
- If the stoppers are together, call 1-800-828-2088 for help.
What if the stoppers do not stay together after mixing?
- The Pen Needle may not be on correctly.
- Make sure that the Pen Needle is on the right way and that the threads are lined up. Be sure that the needle is firmly attached. You may need to use a new Pen Needle.
What if I have more than a few drops on the tip of the Pen Needle after injection?
This may mean that you did not hold the needle in your thigh for the full 10 seconds. When you give your next scheduled injection, be sure that you hold the needle in your thigh for at least10 seconds.
What should I do if there are many small bubbles after mixing the NATPARA medicine cartridge?
It is normal to see small air bubbles in the liquid after you finish mixing your NATPARA medicine.
What should I do if the liquid is colored?
Call 1-800-828-2088 for help.
What should I do if the liquid has small particles in it?
It is normal to sometimes see small particles.
How do I clean my Q-Cliq pen and mixing device?
If needed, clean the Q-Cliq pen and mixing device by wiping them with a damp cloth.
Do not place the Q-Cliq pen and mixing device in water, or wash them with any liquid, such as alcohol.
Can I reuse the Pen Needle?
Do not reuse your Pen Needle. You must use a new Pen Needle for each injection.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
USA
1-800-828-2088
U.S. License Number 1898
NATPARA® is a registered trademark of Takeda Pharmaceuticals U.S.A., Inc.
Q-Cliq® is a registered trademark of Takeda Pharmaceuticals U.S.A., Inc.
©2023 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 2/2023
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
NATPARA is supplied as a multiple-dose, dual-chamber glass cartridge containing a sterile powder and diluent in 4 dosage strengths.
|
For injection: |
25 mcg per dose strength |
(0.4 mg for reconstitution with 1.13 mL) |
|
For injection: |
50 mcg per dose strength |
(0.8 mg for reconstitution with 1.13 mL) |
|
For injection: |
75 mcg per dose strength |
(1.21 mg for reconstitution with 1.13 mL) |
|
For injection: |
100 mcg per dose strength |
(1.61 mg for reconstitution with 1.13 mL) |
NATPARA is supplied as a multiple-dose, dual-chamber glass cartridge containing a sterile lyophilized powder and a sterile diluent for reconstitution in four dosage strengths. (3)
For injection: 25 mcg, 50 mcg, 75 mcg, or 100 mcg. (3)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
NATPARA (parathyroid hormone) for injection for subcutaneous use is supplied as a medication cartridge, which is comprised of a multiple-dose, dual-chamber glass cartridge containing a sterile lyophilized powder and a sterile diluent, within a plastic cartridge holder. The medication cartridge is available in 4 dosage strengths (25, 50, 75, and 100 mcg/dose). The 25 mcg/dose cartridge contains 0.4 mg parathyroid hormone; the 50 mcg/dose cartridge contains 0.8 mg parathyroid hormone; the 75 mcg/dose cartridge contains 1.21 mg parathyroid hormone; the 100 mcg/dose cartridge contains 1.61 mg parathyroid hormone.
NATPARA is supplied in the following packages:
- 2 cartridges of 25 mcg/dose strength (NDC 68875-0202-2)
- 2 cartridges of 50 mcg/dose strength (NDC 68875-0203-2)
- 2 cartridges of 75 mcg/dose strength (NDC 68875-0204-2)
- 2 cartridges of 100 mcg/dose strength (NDC 68875-0205-2)
The disposable NATPARA medication cartridge is designed for use with a reusable mixing device for product reconstitution and a reusable Q-Cliq pen injector for drug delivery. The Q-Cliq pen is designed to deliver a fixed volumetric dose of 71.4 µL. Using the Q-Cliq pen, each NATPARA medication cartridge delivers 14 doses; each dose contains 25, 50, 75, or 100 mcg of NATPARA depending on the product dosage strength.
Designed for use with 31G × 8 mm BD Ultra-Fine™ Pen Needles.
The mixing device, provided in a separate carton, is designed to enable reconstitution of the product before the first use of each cartridge. The mixing device can be used to reconstitute up to 6 NATPARA medication cartridges.
The Q-Cliq pen, packaged in a separate carton, can be used for up to 2 years of daily treatment by replacing the reconstituted cartridge every two weeks (14 days).
Instructions for use of the mixing device and the Q-Cliq pen are provided with the NATPARA medication cartridges.
16.2 Storage and Handling
Prior to reconstitution, the dual-chamber NATPARA medication cartridge should be stored in the package provided at refrigerated temperature, 36 to 46°F (2 to 8°C). After reconstitution, the medication cartridge should be stored in the Q-Cliq pen under refrigeration at 36 to 46°F (2 to 8°C). The reconstituted product can be used for up to 14 days under these conditions. Store away from heat and light. Avoid exposure to elevated temperatures. Discard reconstituted NATPARA medication cartridges after 14 days.
Do not freeze or shake. Do not use NATPARA if it has been frozen or shaken.
The mixing device and empty Q-Cliq pen can be stored at room temperature.
Safely discard needles.