Levothyroxine sodium
These highlights do not include all the information needed to use LEVOTHYROXINE SODIUM TABLETS safely and effectively. See full prescribing information for LEVOTHYROXINE SODIUM TABLETS. LEVOTHYROXINE SODIUM tablets, for oral use Initial U.S. Approval: 2002
0cffbc96-7a02-4538-9966-b69249be6eb2
HUMAN PRESCRIPTION DRUG LABEL
Jul 19, 2023
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Levothyroxine Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 50 mcg Tablets: Label
NDC 68788-7911
Levothyroxine
Sodium Tablets, USP
50 mcg
(0.05 mg)
Rx only

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Serious Risks Related to Overtreatment or Undertreatment with
Levothyroxine Sodium Tablets
Levothyroxine sodium tablets has a narrow therapeutic index. Overtreatment or undertreatment with levothyroxine sodium tablets may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, gastrointestinal function, and glucose and lipid metabolism in adult or pediatric patients.
In pediatric patients with congenital and acquired hypothyroidism, undertreatment may adversely affect cognitive development and linear growth, and overtreatment is associated with craniosynostosis and acceleration of bone age [Use in Specific Populations (8.4)].
Titrate the dose of levothyroxine sodium tablets carefully and monitor response to titration to avoid these effects [see Dosage and Administration (2.4)]. Consider the potential for food or drug interactions and adjust the administration or dosage of levothyroxine sodium tablets as needed [see Dosage and Administration (2.1), Drug Interactions (7.1), and Clinical Pharmacology (12.3)].
5.2 Cardiac Adverse Reactions in the Elderly and in Patients with
Underlying Cardiovascular Disease
Over-treatment with levothyroxine may cause an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias, particularly in patients with cardiovascular disease and in elderly patients. Initiate levothyroxine sodium therapy in this population at lower doses than those recommended in younger individuals or in patients without cardiac disease [see Dosage and Administration (2.3), Use in Specific Populations (8.5)] .
Monitor for cardiac arrhythmias during surgical procedures in patients with coronary artery disease receiving suppressive levothyroxine sodium therapy. Monitor patients receiving concomitant levothyroxine sodium and sympathomimetic agents for signs and symptoms of coronary insufficiency.
If cardiac symptoms develop or worsen, reduce the levothyroxine sodium tablets dose or withhold for one week and restart at a lower dose.
5.3 Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism and may result in unpredictable absorption of levothyroxine sodium from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
5.4 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with levothyroxine sodium [see Contraindications (4)] .
5.5 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing levothyroxine sodium [see Drug Interactions (7.2)] .
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-
Replacement
Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in post-menopausal women. The increased bone resorption may be associated with increased serum levels and urinary excretion of calcium and phosphorous, elevations in bone alkaline phosphatase, and suppressed serum parathyroid hormone levels. Administer the minimum dose of levothyroxine sodium that achieves the desired clinical and biochemical response to mitigate this risk.
5.7 Risk of Allergic Reactions Due to Tartrazine
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic- type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
•
Serious risks related to overtreatment or undertreatment with levothyroxine sodium tablets: Titrate the dose of levothyroxine sodium carefully and monitor response to titration. (5.1)
•
Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate levothyroxine sodium at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation. (2.3, 5.2, 8.5)
•
Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.3)
•
Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of levothyroxine sodium treatment. (5.4)
•
Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy. (5.5)
•
Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose. (5.6)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage ( 1) |
8/2022 |
|
Dosage and Administration ( 2.2) |
8/2022 |
|
Dosage and Administration ( 2.3) |
8/2022 |
|
Warnings and Precautions ( 5.1) |
8/2022 |
|
Warnings and Precautions ( 5.4) |
8/2022 |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
All tablets having functional scoring
Levothyroxine sodium tablets USP are available as follows (Table 4):
Table 4: Levothyroxine Sodium Tablet Strengths and Identifying Features|
Tablet Strength |
Tablet Description |
|
25 mcg |
Round shaped, Orange colored, uncoated tablets, break line on both side and debossed with "P" and "1" on one side and plain on other side. |
|
50 mcg |
Round shaped, White colored, uncoated tablets, break line on both side and debossed with "P" and "2" on one side and plain on other side. |
|
75 mcg |
Round shaped, Violet colored, uncoated tablets, break line on both side and debossed with "P" and "3" on one side and plain on other side. |
|
88 mcg |
Round shaped, Olive colored, uncoated tablets, break line on both side and debossed with "P" and "4" on one side and plain on other side. |
|
100 mcg |
Round shaped, Yellow colored, uncoated tablets, break line on both side and debossed with "P" and "14" on one side and plain on other side. |
|
112 mcg |
Round shaped, Rose colored, uncoated tablets, break line on both side and debossed with "P" and "6" on one side and plain on other side. |
|
125 mcg |
Round shaped, Brown colored, uncoated tablets, break line on both side and debossed with "P" and "7" on one side and plain on other side. |
|
137 mcg |
Round shaped, Turquoise colored, uncoated tablets, break line on both side and debossed with "P" and "8" on one side and plain on other side. |
|
150 mcg |
Round shaped, Blue colored, uncoated tablets, break line on both side and debossed with "P" and "9" on one side and plain on other side. |
|
175 mcg |
Round shaped, Lilac colored, uncoated tablets, break line on both side and debossed with "P" and "10" on one side and plain on other side. |
|
200 mcg |
Round shaped, Pink colored, uncoated tablets, break line on both side and debossed with "P" and "11" on one side and plain on other side. |
|
300 mcg |
Round shaped, Green colored, uncoated tablets, break line on both side and debossed with "P" and "12" on one side and plain on other side. |
Tablets (Functional Scoring): 25, 50, 75, 88, 100, 112, 125, 137, 150, 175, 200, and 300 mcg (3)
OVERDOSAGE SECTION
10 OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)] . In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures occurred in a 3-year-old child ingesting 3.6 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Reduce the levothyroxine sodium dosage or discontinue temporarily if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient’s medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
DESCRIPTION SECTION
11 DESCRIPTION
Levothyroxine sodium tablets, USP is L-thyroxine (T4) and contains synthetic crystalline L-3,3’,5,5’-tetraiodothyronine sodium salt. Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C 15H 10I 4N NaO 4• H 2O, molecular weight of 798.86 (anhydrous), and structural formula as shown:
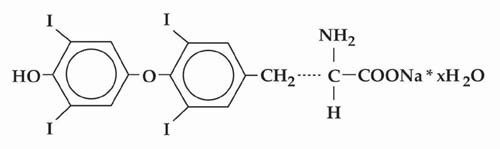
Levothyroxine sodium tablets, USP for oral administration are supplied in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg, and 300 mcg. Each levothyroxine sodium tablet contains the inactive ingredients microcrystalline sodium, light magnesium oxide, sodium starch glycolate and sodium stearyl fumarate.
Levothyroxine sodium tablets, USP contain no ingredients made from a gluten- containing grain (wheat, barley, or rye). Table 9 provides a listing of the color additives by tablet strength:
Table 9. Levothyroxine Sodium Tablet Color Additives|
Strength |
Color additive(s) |
|
25 |
FD&C Yellow No. 6 Aluminum Lake |
|
50 |
None |
|
75 |
FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake |
|
88 |
FD&C Blue No. 2 Aluminum Lake, FD&C Yellow No. 5 Aluminum Lake |
|
100 |
FD&C Yellow No. 5 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake |
|
112 |
FD&C Red No. 40 Aluminum Lake, Carmine |
|
125 |
FD&C Yellow No. 6 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake |
|
137 |
FD&C Blue No. 1 Aluminum Lake |
|
150 |
FD&C Blue No. 2 Aluminum Lake |
|
175 |
FD&C Blue No. 1 Aluminum Lake, Carmine |
|
200 |
FD&C Red No. 40 Aluminum Lake |
|
300 |
D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake |
Levothyroxine sodium tablets, USP meets USP Dissolution Test 7.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals to evaluate the carcinogenic potential of levothyroxine have not been performed. Studies to evaluate mutagenic potential and animal fertility have not been performed.
