ChiRhoStim
These highlights do not include all the information needed to use CHIRHOSTIM® safely and effectively. See full prescribing information for CHIRHOSTIM®. CHIRHOSTIM® (human secretin) for injection, for intravenous use Initial U.S. Approval: 2004
b4dfe70f-0a86-4488-9ba1-dcd8f86cbfcd
HUMAN PRESCRIPTION DRUG LABEL
Oct 14, 2021
ChiRhoClin, Inc.
DUNS: 036663672
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Human Secretin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Human Secretin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
ChiRhoStim ® is indicated for the stimulation of:
- pancreatic secretions, including bicarbonate, to aid in the diagnosis of pancreatic exocrine dysfunction.
- gastrin secretion to aid in the diagnosis of gastrinoma, and
- pancreatic secretions to facilitate the identification of the ampulla of Vater and accessory papilla during endoscopic retrograde cholangiopancreatography (ERCP).
- ChiRhoStim® is a secretin class hormone indicated for stimulation of:
- pancreatic secretions, including bicarbonate, to aid in the diagnosis of exocrine pancreas dysfunction (1)
- gastrin secretion to aid in the diagnosis of gastrinoma (1)
- pancreatic secretions to facilitate the identification of the ampulla of Vater and the accessory papilla during endoscopic retrograde cholangiopancreatography (ERCP) (1)
(1)
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
None.
None (4) (4)
WARNINGS AND PRECAUTIONS SECTION
WARNINGS AND PRECAUTIONS
Patients with alcoholic or other liver disease may be hyperresponsive to stimulation with ChiRhoStim®, masking the presence of coexisting pancreatic disease. Consider additional testing and clinical assessments for aid in diagnosis.
- Hyporesponse to Secretin Stimulation Testing in Patients with Vagotomy, Inflammatory Bowel Disease or Receiving Anticholinergics: Discontinue anticholinergic drugs at least 5 half-lives prior to stimulation testing; consider additional testing and clinical assessments for aid in diagnosis. (2.1, 5.1, 7.1)
- Hyperresponse to Secretin Stimulation Testing: Increased gastrin secretion in patients receiving H 2-receptor antagonists or PPIs falsely suggesting gastrinoma; discontinue co-administered drug prior to stimulation testing. Increased pancreatic secretions in patients with alcoholic or other liver disease masking coexisting pancreatic disease; consider additional testing and clinical assessments for aid in diagnosis. (2.1, 5.2, 7.2)
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed during the clinical trials of a drug cannot always be directly compared to the rates observed during the clinical trials of another drug and may not reflect the adverse reaction rates observed in practice.
The data described below reflect exposure to ChiRhoStim® in 531 patients from an open-label clinical trial. The population consisted of patients aged 1 to 91 years, 185 males, 346 females, 480 Caucasians, 31 Blacks, 12 American Indians, 6 Hispanics, and 2 Asians with known or suspected diseases of the exocrine pancreas including chronic pancreatitis and pancreatic cancer. Most patients received a single dose of ChiRhoStim® in a dose range of 0.2 mcg/kg to 0.4 mcg/kg. The most common adverse reactions (reported in at least 2 patients in the trial) are listed in Table 2.
TABLE 2
Adverse Reactions in at Least 2 Patients Treated with a Single-Dose of ChiRhoStim® in a Clinical Trial
|
Adverse Reaction |
ChiRhoStim® Number of Patients N = 531 |
|
Nausea |
9 |
|
Vomiting |
3 |
|
Flushing |
2 |
|
Upset stomach |
2 |
Most common adverse reactions (≥2 patients) are nausea, vomiting, flushing, and upset stomach. (6.1) (6)
(6)
To report SUSPECTED ADVERSE REACTIONS, contact ChiRhoClin, Inc. at 1-877-272-4888 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch. (6)
(6)
See 17 for PATIENT COUNSELING INFORMATION. (6)
(6)
Revised: 07/2017 (6)
DRUG INTERACTIONS SECTION
DRUG INTERACTIONS
7.1 Hyporesponse with Anticholinergics
The concomitant use anticholinergic drugs may cause a hyporesponse to stimulation testing with ChiRhoStim ®. Discontinue anticholinergic drugs at least 5 half-lives before administering ChiRhoStim ®[see Dosage and Administration (2.1)].
7.2 Hyperresponse of Gastrin Secretion with H2-Receptor Antagonists and PPIs
The concomitant use of H 2-receptor antagonists or PPIs may cause a hyperresponse in gastrin secretion in response to stimulation testing with ChiRhoStim ®, falsely suggesting gastrinoma.
Discontinue H 2-receptor antagonists at least 2 days before administering ChiRhoStim ® to aid in the diagnosis of gastrinoma.
The time it takes for serum gastrin concentrations to return to baseline following discontinuation of PPIs is specific to the individual drug. Consult the prescribing information of each specific PPI before administering ChiRhoStim ® to aid in the diagnosis of gastrinoma.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Dosage and Administration (2.1) 07/2017
Contraindications, removed (4) 07/2017
Warnings and Precautions (5.1, 5.2) 07/2017
DOSAGE FORMS & STRENGTHS SECTION
DOSAGE FORMS AND STRENGTHS
For injection: 16 mcg or 40 mcg of human secretin as a white lyophilized powder in single-dose vial for reconstitution.
For injection: 16 mcg or 40 mcg of human secretin as a lyophilized powder in single-dose vial for reconstitution (3) (3)
USE IN SPECIFIC POPULATIONS SECTION
8.1 Pregnancy
Risk Summary
There are no available data (either clinical studies or postmarketing reports) of use of synthetic human secretin in pregnant women. Animal reproduction studies have not been conducted with synthetic human secretin.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of synthetic human secretin in human or animal milk, the effects of synthetic human secretin on the breastfed infant, or the effects of synthetic human secretin on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ChiRhoStim ® and any potential adverse effects on the breastfed infant from ChiRhoStim ® or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ChiRhoStim ® in pediatric patients have not been established.
8.5 Geriatric Use
Among the 531 patients who have received ChiRhoStim ® in a clinical trial, 11% were 65 years of age or older and 5% were 75 years of age or older. No overall differences in safety, pharmacologic response, or diagnostic effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and the younger patients, but greater sensitivity of some older individuals cannot be ruled out.
DESCRIPTION SECTION
DESCRIPTION
ChiRhoStim ® is a pure sterile, nonpyrogenic, lyophilized white cake powder acetate salt of secretin, a peptide hormone. ChiRhoStim ® has an amino acid sequence identical to the naturally occurring hormone consisting of 27 amino acids. Synthetic human secretin is chemically defined as follows:
Molecular Weight 3039.44
Empirical Formula: C 130H 220N 44O 39
CAS # 108153-74-8
Structural Formula:
His-Ser-Asp-Gly-Thr-Phe-Thr-Ser-Glu-Leu-Ser-Arg-Leu-Arg-Glu-Gly-Ala-Arg-Leu- Gln-Arg-Leu-Leu-Gln-Gly-Leu-Val-NH 2
The standard unit of biological activity for ChiRhoStim ® is the clinical unit (CU). (3) One (1) CU of secretin biological activity is equal to 0.2 micrograms (mcg) of human secretin.
ChiRhoStim ® is available in two strengths:
As a 10 mL single-dose vial which contains 16 mcg of purified synthetic human secretin, 1.5 mg of L-cysteine hydrochloride, 20 mg of mannitol, and 9 mg of sodium chloride. When reconstituted in 8 mL of Sodium Chloride Injection USP, each mL of solution contains 2 mcg synthetic human secretin for intravenous use. The pH of the reconstituted solution has a range of 3 to 6.5.
As a 10 mL single-dose vial which contains 40 mcg of purified synthetic human secretin, 3.75 mg of L-cysteine hydrochloride, 50 mg of mannitol, and 22.5 mg of sodium chloride per vial. When reconstituted in 10 mL of Sodium Chloride Injection USP, each mL of solution contains 4 mcg synthetic human secretin for intravenous use. The pH of the reconstituted solution has a range of 3 to 6.5.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Mechanism of Action
12.1 Mechanism of Action
The primary action of ChiRhoStim ® is to stimulate pancreatic ductal cells to secrete pancreas fluid in large volumes that contain bicarbonate.
Secretin is a hormone that is normally released from the duodenum upon exposure of the proximal intestinal lumen to gastric acid, fatty acids and amino acids. Secretin is released from enterochromaffin cells in the intestinal mucosa. Secretin receptors have been identified in the pancreas, stomach, liver, colon and other tissues. When secretin binds to secretin receptors on pancreatic duct cells it opens cystic fibrosis transmembrane conductance regulator (CFTR) channels, leading to secretion of bicarbonate- rich-pancreatic fluid. Secretin may also work through vagal-vagal neural pathways since stimulation of the efferent vagus nerve stimulates bicarbonate secretion and atropine blocks secretin-stimulated pancreatic secretion.
Pharmacokinetics
12.3 Pharmacokinetics
The pharmacokinetic profile for synthetic human secretin was evaluated in 12 healthy subjects following a single-dose of human secretin administered as a 0.4 mcg/kg intravenous bolus. The plasma concentrations of human secretin declined to baseline concentrations within 90 to 120 minutes. The elimination half-life of synthetic human secretin is 45 minutes. The clearance of synthetic human secretin is 580.9 ± 51.3 mL/min and the volume of distribution is 2.7 liters.
CLINICAL STUDIES SECTION
CLINICAL STUDIES
14.1 Stimulation of Pancreatic Secretions, Including Bicarbonate to Aid in
the Diagnosis of Exocrine Pancreas Dysfunction
ChiRhoStim® administered intravenously stimulates the exocrine pancreas to
secrete pancreatic juice, which can assist in the diagnosis of exocrine
pancreas dysfunction. Normal ranges for pancreatic secretory response to
intravenous secretin in patients with defined pancreatic disease have been
shown to vary. One source of variation is related to the inter-investigator
differences in operative technique.
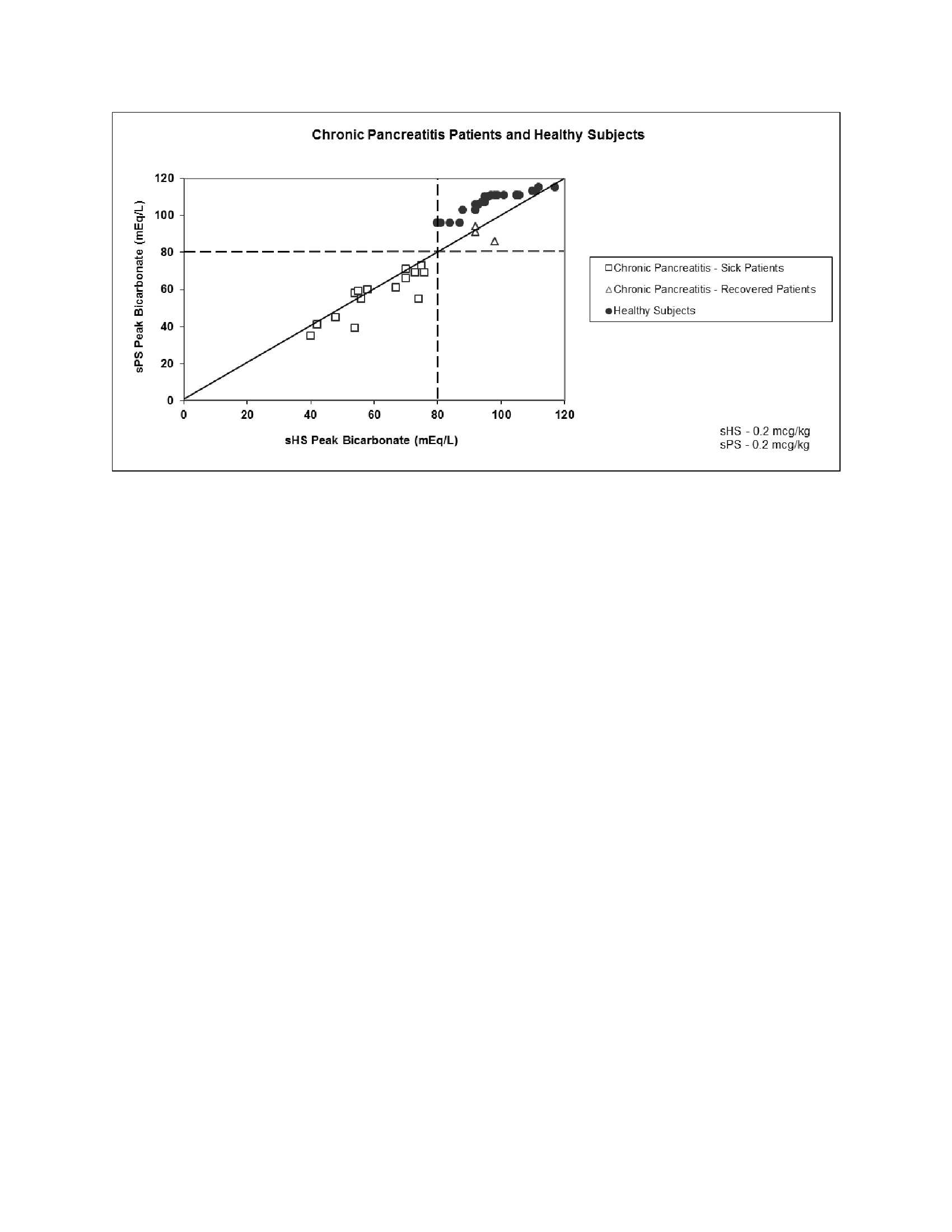
In two studies, a total of 18 patients with a documented history of chronic pancreatitis were given 0.2 mcg/kg synthetic human secretin (sHS), 0.2 mcg/kg synthetic porcine secretin (sPS), and 1 CU/kg (equal to 0.2 mcg/kg for biologically derived secretin (bPS)) in a crossover design. The results appear in Figures 1 and 2. In another study, 35 healthy subjects were given sHS at a dose of 0.2 mcg/kg. The results appear in Figures 1 and 2.
FIGURE 1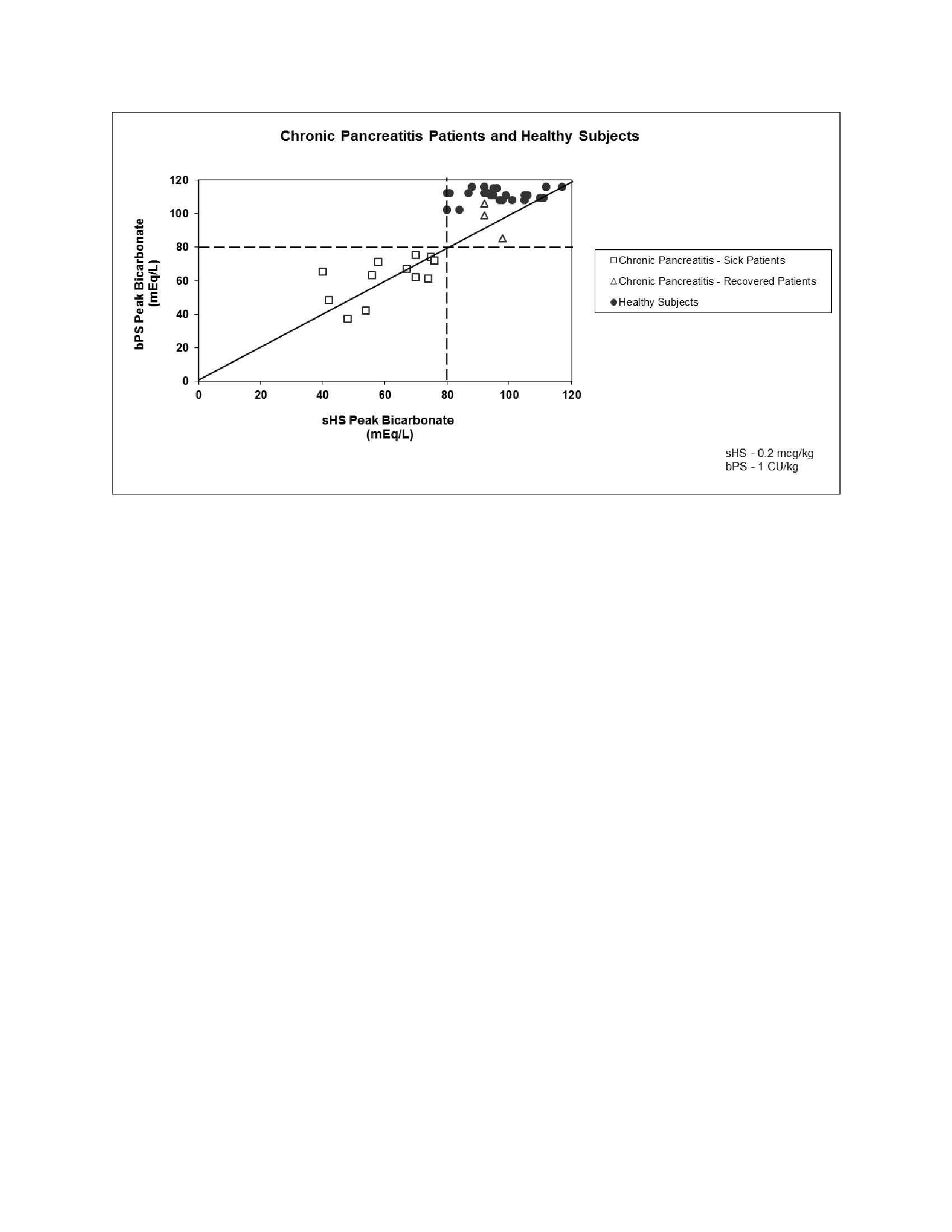 FIGURE 2
FIGURE 2
 The values
obtained for Figures 1 and 2 were performed by investigators skilled in
performing secretin stimulation testing and are to be taken only as
guidelines. These results should not be generalized to results of secretin
stimulation testing conducted in other laboratories. However, a volume
response of less than 2 mL/kg/hr, bicarbonate concentration of less than 80
mEq/L, and a bicarbonate output of less than 0.2 mEq/kg/hr are consistent with
impaired pancreatic function.
The values
obtained for Figures 1 and 2 were performed by investigators skilled in
performing secretin stimulation testing and are to be taken only as
guidelines. These results should not be generalized to results of secretin
stimulation testing conducted in other laboratories. However, a volume
response of less than 2 mL/kg/hr, bicarbonate concentration of less than 80
mEq/L, and a bicarbonate output of less than 0.2 mEq/kg/hr are consistent with
impaired pancreatic function.
A physician or institution planning to perform secretin stimulation testing as an aid to the diagnosis of pancreatic disease should begin by assessing enough normal subjects (greater than 5) to develop proficiency in proper techniques and to generate normal response ranges for the commonly assessed parameters for pancreatic exocrine response to ChiRhoStim®.
In three crossover studies evaluating 21 different patients with a documented history of chronic pancreatitis, sHS was compared to sPS and bPS at a dose of 0.2 mcg/kg for each drug. All of the patients treated with these drugs had peak bicarbonate concentrations of less than 80 mEq/L.
Pancreatic secretory response to intravenous synthetic human secretin in 35 healthy subjects demonstrated a mean peak bicarbonate concentration of 100 mEq/L and a mean total volume over one hour of 260.7 mL. All 35 subjects had peak bicarbonate concentrations greater than or equal to 80 mEq/L.
14.2 Stimulation of Gastrin Secretion to Aid in the Diagnosis of Gastrinoma
ChiRhoStim® administered intravenously stimulates gastrin release in patients with gastrinoma (Zollinger-Ellison Syndrome), whereas no or only small changes in serum gastrin concentrations occur in healthy subjects and in patients with duodenal ulcer disease. Discriminant analysis was used to establish secretin stimulation testing as an aid in the diagnosis of gastrinoma. An increase from basal levels of greater than or equal to 110 pg/mL was the optimal point separating positive and negative tests. This gastrin response is the basis for the use of secretin as a provocative test in the evaluation of patients in whom gastrinoma is a diagnostic consideration.
In a three way crossover study, 6 patients with tissue confirmed gastrinoma received synthetic human secretin (ChiRhoStim®), synthetic porcine secretin and biologically derived porcine secretin at a dose of 0.4 mcg/kg for each drug. Serum gastrin levels were reported to be greater than 110 pg/mL for all secretin products tested after stimulation. Testing of ChiRhoStim® in 12 healthy subjects demonstrated completely negative results for gastrinoma.
14.3 Stimulation of Pancreatic Secretion to Facilitate Identification of the Ampulla of Vater and the Accessory Papilla During Endoscopic Retrograde Cholangiopancreatography (ERCP)
In a randomized, placebo controlled crossover study in 24 patients with pancreas divisum undergoing ERCP, ChiRhoStim® at a dose of 0.2 mcg/kg resulted in 16 of 24 successful cannulations of the minor duct compared to 2 of 24 for placebo.
REFERENCES SECTION
REFERENCES
- Gardner TB, Purich ED and Gordon SR. Pancreatic Duct Compliance After Secretin Stimulation. A Novel Endoscopic Ultrasound Diagnostic Tool for Chronic Pancreatitis. Pancreas. 2012 Mar;41(2):290-94.
- Jorpes, E. and Mutt V. On the biological assay of secretin. The reference standard. Acta Physiol Scand. 1966 Mar;66(3):316-25.
HOW SUPPLIED SECTION
HOW SUPPLIED/STORAGE AND HANDLING
ChiRhoStim ® (human secretin), for injection is supplied as a white lyophilized sterile powder in a single-dose vial for reconstitution:
NDC # 67066-005-01 16 mcg
NDC # 67066-007-01 40 mcg
Store at -20°C (freezer). Protect from light.
PATIENT MEDICATION INFORMATION SECTION
PATIENT COUNSELING INFORMATION
Advise the patient to tell their healthcare provider all the medications they are taking, including anticholinergic drugs, H 2-receptor antagonists or PPIs [see Warnings and Precautions (5.1, 5.2)].
Manufactured for:
ChiRhoClin, Inc.
Burtonsville, MD 20866-6129
005PI507
