Gum Detoxify
5820068 Meijer Gum Detoxify
0338495b-08a5-047f-e063-6394a90aa124
HUMAN OTC DRUG LABEL
Aug 12, 2025
Meijer, Inc.
DUNS: 006959555
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Stannous Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
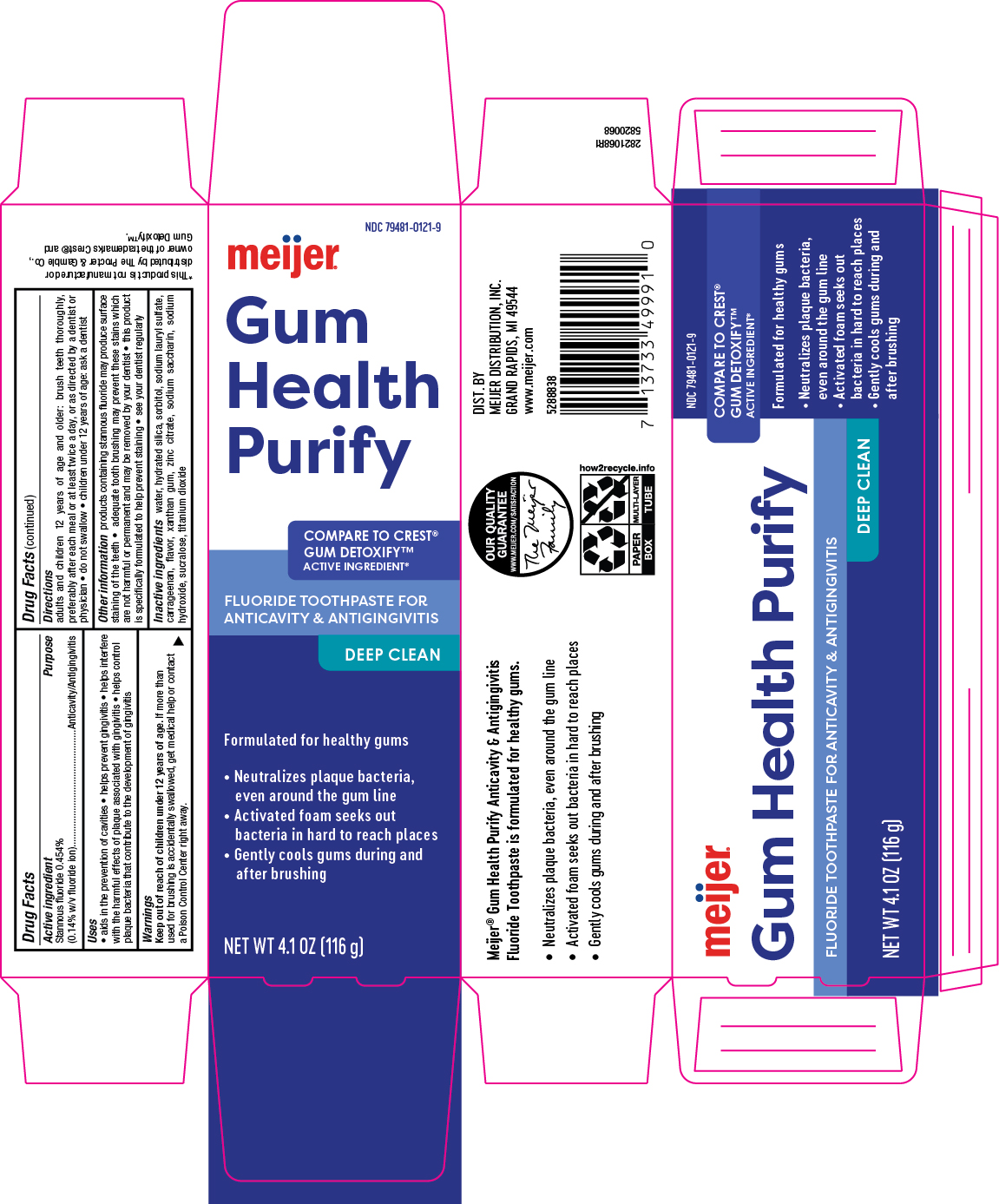
INDICATIONS & USAGE SECTION
Uses
aids in the prevention of cavities * helps prevent gingivitis * helps interfere with teh harmful effects of plaque associated with gingivitis * helps control plaque bacteria that contribute to teh development of gingivitis
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Stannous fluoride 0.454% (0.14% w/v fluoride ion)
OTC - PURPOSE SECTION
Purpose
Anticavity/Antigingivitis
WARNINGS SECTION
Warnings
Keep out of reach of children under 12 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children under 12 years of age
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DOSAGE & ADMINISTRATION SECTION
Directions
adults and children 12 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician * do not swallow * children under 12 years of age: ask a dentist
OTHER SAFETY INFORMATION
Other information
products containing stannous fluoride may produce surface staining of the teeth * adequate tooth brushing may prevent these stains which are not harmful or permanent and may be removed by your dentist * this product is specifically formulated to help prevent staining * see your dentist regularly
INACTIVE INGREDIENT SECTION
Inactive ingredients
water, hydrated silica, sorbitol, sodium lauryl sulfate, carrageenan, flavor, xanthan gum, zinc citrate, sodium saccharin, sodium hydroxide, sucralose, titanium dioxide
