ATROPINE SULFATE
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for Initial U.S. Approval: 1960
d5e07279-48ca-4f83-9633-299e65a7f821
HUMAN PRESCRIPTION DRUG LABEL
Mar 16, 2023
Accord Healthcare Inc.
DUNS: 604222237
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ATROPINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
ATROPINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
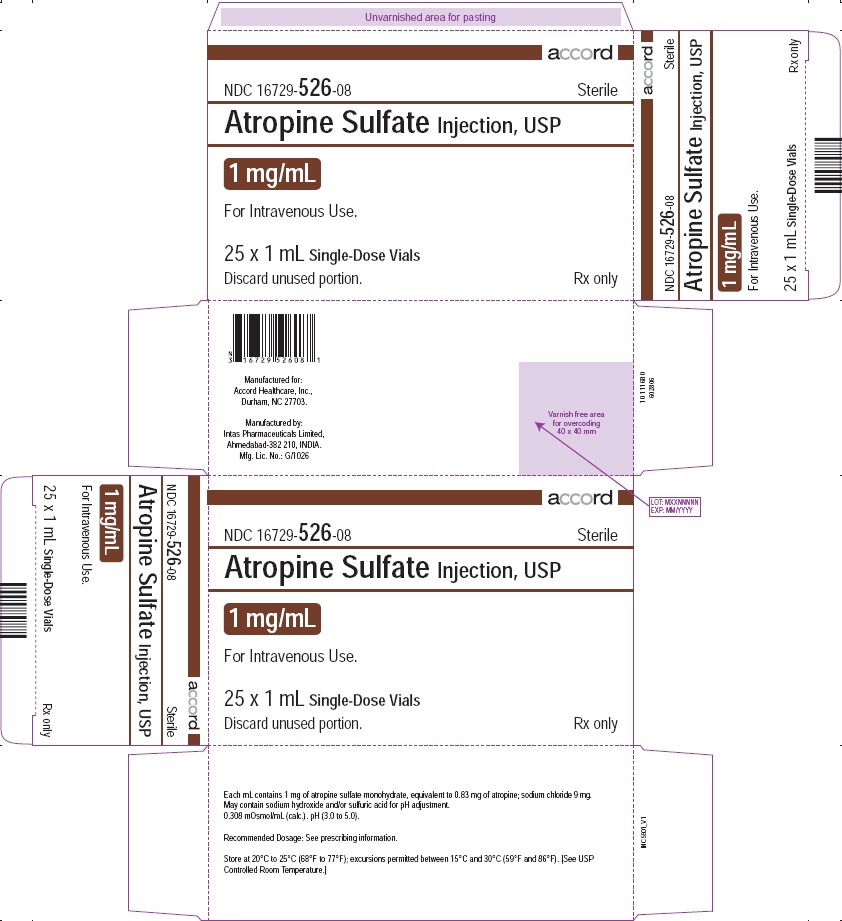
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Atropine Sulfate Injection, USP 1 mg/mL 25 Vials
Carton
NDC 16729-526-08
Sterile
Atropine Sulfate Injection, USP
1 mg/mL
For Intravenous Use.
25 x 1 mL Single-Dose Vials
Rx only
Discard unused portion
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Tachycardia
When the recurrent use of atropine is essential in patients with coronary artery disease, the total dose should be restricted to 2 to 3 mg (maximum 0.03 to 0.04 mg/kg) to avoid the detrimental effects of atropine-induced tachycardia on myocardial oxygen demand.
5.2 Acute Glaucoma
Atropine may precipitate acute glaucoma.
5.3 Pyloric Obstruction
Atropine may convert partial organic pyloric stenosis into complete obstruction.
5.4 Complete Urinary Retention
Atropine may lead to complete urinary retention in patients with prostatic hypertrophy.
5.5 Viscid Plugs
Atropine may cause inspissation of bronchial secretions and formation of viscid plugs in patients with chronic lung disease.
- Tachycardia ( 5.1)
- Glaucoma ( 5.2)
- Pyloric obstruction ( 5.3)
- Worsening urinary retention ( 5.4)
- Viscid bronchial plugs ( 5.5)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer unless solution is clear and seal is intact. Each vial is intended for single dose only. Discard unused portion.
For intravenous administration.
Titrate based on heart rate, PR interval, blood pressure and symptoms.
2.2 Adult Dosage
Table 1: Recommended Dosage|
Use |
Dose (adults) |
Repeat |
|
Antisialagogue or other antivagal |
0.5 to 1 mg |
1-2 hours |
|
Organophosphorus or muscarinic mushroom poisoning |
2 to 3 mg |
20-30 minutes |
|
Bradyasystolic cardiac arrest |
1 mg |
3-5 minutes; 3 mg maximum total dose |
2.3 Pediatric Dosage
Dosing in pediatric populations has not been well studied. Usual initial dose is 0.01 to 0.03 mg/kg.
2.4 Dosing in Patients with Coronary Artery Disease
Limit the total dose of atropine sulfate to 0.03 to 0.04 mg/kg [see Warnings and Precautions (5.1)].
- For intravenous administration ( 2.1)
- Titrate according to heart rate, PR interval, blood pressure and symptoms ( 2.1)
- Adult dosage
- Antisialagogue or for antivagal effects: Initial single dose of 0.5 to 1 mg ( 2.2)
- Antidote for organophosphorus or muscarinic mushroom poisoning: Initial single dose of 2 to 3 mg, repeated every 20-30 minutes ( 2.2)
- Bradyasystolic cardiac arrest: 1 mg dose, repeated every 3-5 minutes if asystole persists ( 2.2)
- Patients with Coronary Artery Disease: Limit the total dose to 0.03 to 0.04 mg/kg ( 2.4)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed to evaluate the carcinogenic or mutagenic potential of atropine or its potential to affect fertility adversely.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Injection, USP 0.4 and 1 mg/mL are supplied in 1 mL, single- dose glass vials as follows:
Table 2: How Supplied
|
Concentration (mg/mL) |
Package Size |
NDC # |
|
0.4 mg/mL |
1 Vial |
16729-525-63 |
|
10 Vials |
16729-525-03 | |
|
25 Vials |
16729-525-08 | |
|
1 mg/mL |
1 Vial |
16729-526-63 |
|
10 Vials |
16729-526-03 | |
|
25 Vials |
16729-526-08 |
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.]
Manufactured For:
Accord Healthcare, Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.
Manufactured By:
Intas Pharmaceuticals Limited,
Plot No.: 457, 458,
Village – Matoda,
Bavla Road, Ta. - Sanand,
Dist.- Ahmedabad – 382 210.
India.
10 4020 1 6014604
September 2020
