Zozdal Wart Remover
3b387c89-25d5-f12b-e063-6294a90a5942
HUMAN OTC DRUG LABEL
Jul 31, 2025
Jiashen International Trade Limited
DUNS: 989067943
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
WART REMOVER SALICYLIC ACID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
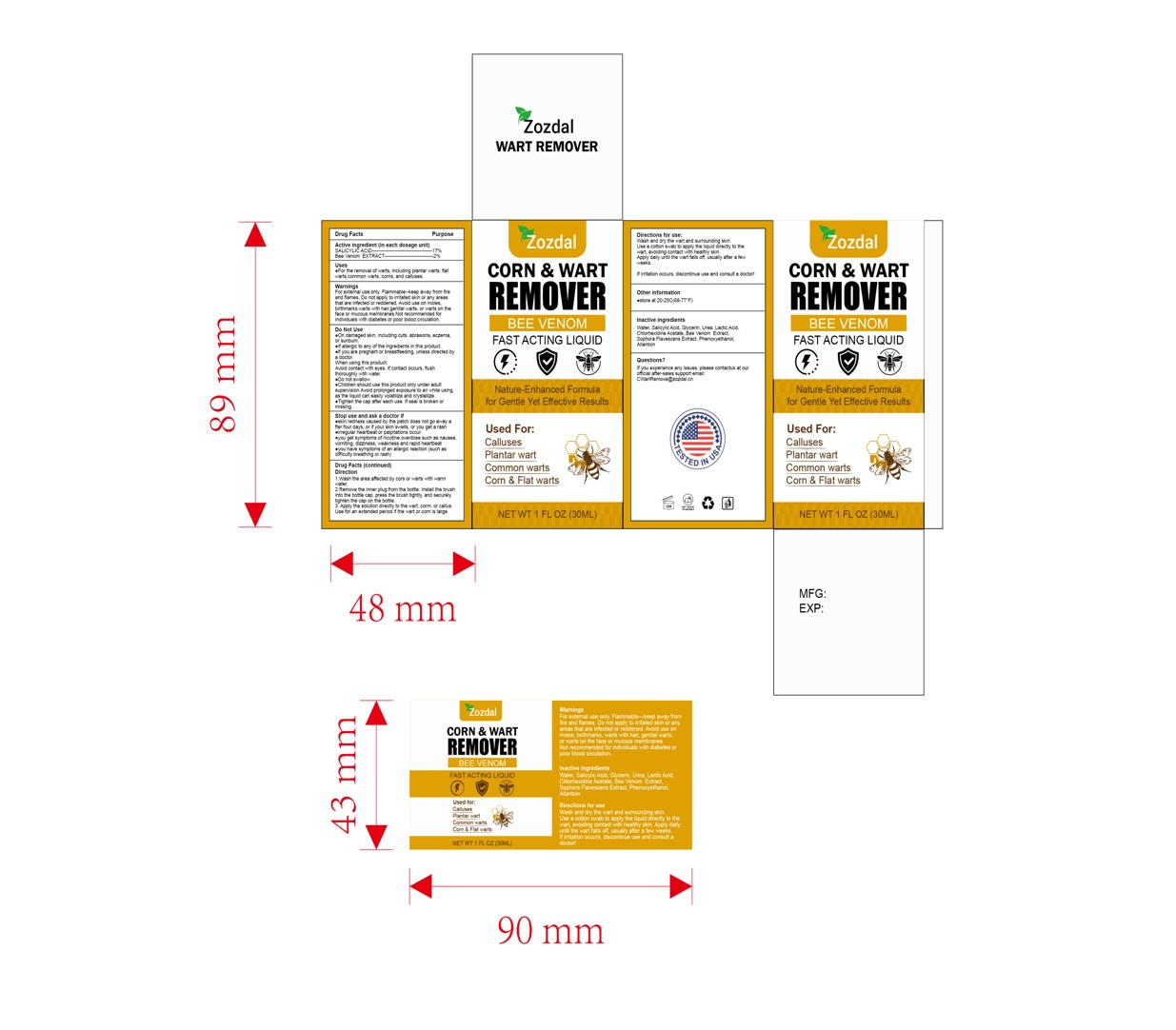
INDICATIONS & USAGE SECTION
Direction
Wash he area aeced by ors or watswith wamwaler2.Removethe lmnerplug fiom the
boe. hsathebnushino the boe cap.pes he knush ighy, and secureltghen hiecap
onhe bole.3.Apythesoluion direclyto
the wart, corm, or callus,Use for an extended period if the wart or corn is
large.
OTC - ACTIVE INGREDIENT SECTION
SALICYLIC ACID 17%
Bee Venom EXTRACT 2%
OTC - PURPOSE SECTION
wart, corn and callus remover
WARNINGS SECTION
WarningsFor extemal use only, Flammable-keep away from fireand flames.Do not apply to irritated skin or any areasthat are infected or reddened.Avoid usehair.genital warts.,or warts on theface ormucous membranes.Not recommended forindividuals with diabetes or poor blood circulation.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep the product out of reach of children. lf accidentally ingested, please call the emergency center immediately
OTC - STOP USE SECTION
stop use and aska docdor eskthnredhescaused by the patch does not o aay ater
lour day,or yourshin snl,or yougeta rasheregular heatbeat or palpaons
cuyougetsmiploms of noine overnose such as
nausea.vomiting,dizziness, weakness and rapid heartbeateyou have symptoms of
an allergic reaction (such asdificully breathing or rash)
DOSAGE & ADMINISTRATION SECTION
Apply the solution directly to the wart, corm, or callus.Use for an extended period if the wart or corn is large.
INACTIVE INGREDIENT SECTION
AQUA
GLYCERIN
LACTIC ACID
UREA
Chlorhexidine Acetate
SOPHORA FLAVESCENS EXTRACT
HYDROXYETHYLCELLULOSE
ALLANTOIN
PHENOXYETHANOL
