Amantadine Hydrochloride
AMANTADINE HYDROCHLORIDE ORAL SOLUTION, USP 50 mg/5 mL
97d6bd4a-31ca-4b33-8353-a536835ef1e1
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2025
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Atlantic Biologicals Corps
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
AMANTADINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
AMANTADINE HYDROCHLORIDE SOLUTION

DESCRIPTION SECTION
DESCRIPTION
Amantadine hydrochloride, USP is designated chemically as 1-adamantanamine hydrochloride.
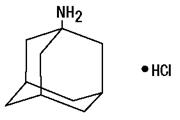
C10H17N • HCl M.W. 187.71
Amantadine hydrochloride is a stable white or nearly white crystalline powder, freely soluble in water and soluble in alcohol and in chloroform.
Amantadine hydrochloride has pharmacological actions as both an anti-Parkinson and an antiviral drug.
Amantadine Hydrochloride Oral Solution, USP contains 50 mg of amantadine hydrochloride per 5 mL and has the following inactive ingredients: anhydrous citric acid, artificial raspberry flavor, methylparaben, propylene glycol, propylparaben, purified water, saccharin sodium, sodium citrate dihydrate, and sorbitol solution.
