Abiraterone acetate
These highlights do not include all the information needed to use ABIRATERONE ACETATE TABLETS safely and effectively. See full prescribing information for ABIRATERONE ACETATE TABLETS. ABIRATERONE ACETATE tablets, for oral use Initial U.S. Approval: 2011
68121a71-5024-4f9e-bd9c-2d412f6a6355
HUMAN PRESCRIPTION DRUG LABEL
Apr 6, 2023
Florida Pharmaceutical Products, LLC
DUNS: 084014259
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Abiraterone acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
NDC 71921-177-06
AbirateroneAcetateTablets****500mg
Rx only
60 film-coated tablets
Florida Pharmaceutical Products, LLC

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions due
to Mineralocorticoid Excess
Abiraterone acetate may cause hypertension, hypokalemia, and fluid retention as a consequence of increased mineralocorticoid levels resulting from CYP17 inhibition [see Clinical Pharmacology (12.1)] . Monitor patients for hypertension, hypokalemia, and fluid retention at least once a month. Control hypertension and correct hypokalemia before and during treatment with abiraterone acetate.
In the combined data from 4 placebo-controlled trials using prednisone 5 mg twice daily in combination with 1,000 mg abiraterone acetate daily, grades 3 to 4 hypokalemia were detected in 4% of patients on the abiraterone acetate arm and 2% of patients on the placebo arm. Grades 3 to 4 hypertension were observed in 2% of patients each arm and grades 3 to 4 fluid retention in 1% of patients each arm.
In LATITUDE (a randomized placebo-controlled, multicenter clinical trial), which used prednisone 5 mg daily in combination with 1,000 mg abiraterone acetate daily, grades 3 to 4 hypokalemia were detected in 10% of patients on the abiraterone acetate arm and 1% of patients on the placebo arm, grades 3 to 4 hypertension were observed in 20% of patients on the abiraterone acetate arm and 10% of patients on the placebo arm. Grades 3 to 4 fluid retention occurred in 1% of patients each arm [see Adverse Reactions (6)] .
Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia or fluid retention, such as those with heart failure, recent myocardial infarction, cardiovascular disease, or ventricular arrhythmia. In postmarketing experience, QT prolongation and Torsades de Pointes have been observed in patients who develop hypokalemia while taking abiraterone acetate.
The safety of abiraterone acetate in patients with left ventricular ejection fraction <50% or New York Heart Association (NYHA) Class III or IV heart failure (in COU-AA-301) or NYHA Class II to IV heart failure (in COU-AA-302 and LATITUDE) has not been established because these patients were excluded from these randomized clinical trials [see Clinical Studies (14)] .
5.2 Adrenocortical Insufficiency
Adrenal insufficiency occurred in 0.3% of 2230 patients taking abiraterone acetate and in 0.1% of 1763 patients taking placebo in the combined data of the 5 randomized, placebo-controlled clinical studies. Adrenocortical insufficiency was reported in patients receiving abiraterone acetate in combination with prednisone, following interruption of daily steroids and/or with concurrent infection or stress.
Monitor patients for symptoms and signs of adrenocortical insufficiency, particularly if patients are withdrawn from prednisone, have prednisone dose reductions, or experience unusual stress. Symptoms and signs of adrenocortical insufficiency may be masked by adverse reactions associated with mineralocorticoid excess seen in patients treated with abiraterone acetate. If clinically indicated, perform appropriate tests to confirm the diagnosis of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations [see Warnings and Precautions (5.1)].
5.3 Hepatotoxicity
In postmarketing experience, there have been abiraterone acetate-associated severe hepatic toxicity, including fulminant hepatitis, acute liver failure and deaths [see Adverse Reactions (6.2)] .
In the combined data of 5 randomized clinical trials, grade 3 to 4 ALT or AST increases (at least 5X ULN) were reported in 6% of 2230 patients who received abiraterone acetate, typically during the first 3 months after starting treatment. Patients whose baseline ALT or AST were elevated were more likely to experience liver test elevation than those beginning with normal values. Treatment discontinuation due to ALT and AST increases or abnormal hepatic function occurred in 1.1% of 2230 patients taking abiraterone acetate. In these clinical trials, no deaths clearly related to abiraterone acetate were reported due to hepatotoxicity events.
Measure serum transaminases (ALT and AST) and bilirubin levels prior to starting treatment with abiraterone acetate, every two weeks for the first three months of treatment and monthly thereafter. In patients with baseline moderate hepatic impairment receiving a reduced abiraterone acetate tablets dose of 250 mg, measure ALT, AST, and bilirubin prior to the start of treatment, every week for the first month, every two weeks for the following two months of treatment and monthly thereafter. Promptly measure serum total bilirubin, AST, and ALT if clinical symptoms or signs suggestive of hepatotoxicity develop. Elevations of AST, ALT, or bilirubin from the patient's baseline should prompt more frequent monitoring. If at any time AST or ALT rise above five times the ULN, or the bilirubin rises above three times the ULN, interrupt abiraterone acetate treatment and closely monitor liver function.
Re-treatment with abiraterone acetate at a reduced dose level may take place only after return of liver function tests to the patient's baseline or to AST and ALT less than or equal to 2.5X ULN and total bilirubin less than or equal to 1.5X ULN [see Dosage and Administration (2.4)] .
Permanently discontinue abiraterone acetate for patients who develop a concurrent elevation of ALT greater than 3 × ULN and total bilirubin greater than 2 × ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation [see Dosage and Administration (2.4)] .
The safety of abiraterone acetate re-treatment of patients who develop AST or ALT greater than or equal to 20X ULN and/or bilirubin greater than or equal to 10X ULN is unknown.
5.4 Increased Fractures and Mortality in Combination with Radium Ra 223
Dichloride
Abiraterone acetate plus prednisone/prednisolone is not recommended for use in combination with radium Ra 223 dichloride outside of clinical trials.
The clinical efficacy and safety of concurrent initiation of abiraterone acetate plus prednisone/prednisolone and radium Ra 223 dichloride was assessed in a randomized, placebo-controlled multicenter study (ERA-223 trial) in 806 patients with asymptomatic or mildly symptomatic castration-resistant prostate cancer with bone metastases. The study was unblinded early based on an Independent Data Monitoring Committee recommendation.
At the primary analysis, increased incidences of fractures (28.6% vs 11.4%) and deaths (38.5% vs 35.5%) have been observed in patients who received abiraterone acetate plus prednisone/prednisolone in combination with radium Ra 223 dichloride compared to patients who received placebo in combination with abiraterone acetate plus prednisone/prednisolone.
5.5 Embryo-Fetal Toxicity
The safety and efficacy of abiraterone acetate have not been established in females. Based on animal reproductive studies and mechanism of action, abiraterone acetate can cause fetal harm and loss of pregnancy when administered to a pregnant female. In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with abiraterone acetate and for 3 weeks after the last dose of abiraterone acetate [see Use in Specific Populations (8.1, 8.3)] . Abiraterone acetate should not be handled by females who are or may become pregnant [see How Supplied/Storage and Handling (16)].
5.6 Hypoglycemia
Severe hypoglycemia has been reported when Abiraterone acetate was administered to patients with preexisting diabetes receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide [see Drug Interactions ( 7.2)]. Monitor blood glucose in patients with diabetes during and after discontinuation of treatment with Abiraterone acetate. Assess if antidiabetic drug dosage needs to be adjusted to minimize the risk of hypoglycemia.
- Mineralocorticoid excess: Closely monitor patients with cardiovascular disease. Control hypertension and correct hypokalemia before treatment. Monitor blood pressure, serum potassium and symptoms of fluid retention at least monthly. ( 5.1)
- Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations. ( 5.2)
- Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue abiraterone acetate tablets dosing as recommended. ( 5.3)
- Increased fractures and mortality in combination with radium Ra 223 dichloride: Use of abiraterone acetate plus prednisone/prednisolone in combination with radium Ra 223 dichloride is not recommended. ( 5.4)
- Embryo-Fetal Toxicity: Abiraterone acetate can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. ( 5.5, 8.1, 8.3)
- Hypoglycemia: Severe hypoglycemia has been reported in patients with pre-existing diabetes who are taking medications containing thiazolidinediones (including pioglitazone) or repaglinide. Monitor blood glucose in patients with diabetes and assess if antidiabetic agent dose modifications are required. ( 5.6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs that Inhibit or Induce CYP3A4 Enzymes
Based on in vitro data, abiraterone acetate is a substrate of CYP3A4.
In a dedicated drug interaction trial, co-administration of rifampin, a strong CYP3A4 inducer, decreased exposure of abiraterone by 55%. Avoid concomitant strong CYP3A4 inducers during abiraterone acetate treatment. If a strong CYP3A4 inducer must be co-administered, increase the abiraterone acetate dosing frequency [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)] .
In a dedicated drug interaction trial, co-administration of ketoconazole, a strong inhibitor of CYP3A4, had no clinically meaningful effect on the pharmacokinetics of abiraterone [see Clinical Pharmacology (12.3)] .
7.2 Effects of Abiraterone on Drug Metabolizing Enzymes
Abiraterone acetate is an inhibitor of the hepatic drug-metabolizing enzymes CYP2D6 and CYP2C8. In a CYP2D6 drug-drug interaction trial, the C max and AUC of dextromethorphan (CYP2D6 substrate) were increased 2.8- and 2.9-fold, respectively, when dextromethorphan was given with abiraterone acetate 1,000 mg daily and prednisone 5 mg twice daily. Avoid co-administration of abiraterone acetate with substrates of CYP2D6 with a narrow therapeutic index (e.g., thioridazine). If alternative treatments cannot be used, consider a dose reduction of the concomitant CYP2D6 substrate drug [see Clinical Pharmacology (12.3)] .
In a CYP2C8 drug-drug interaction trial in healthy subjects, the AUC of pioglitazone (CYP2C8 substrate) was increased by 46% when pioglitazone was given together with a single dose of 1,000 mg abiraterone acetate. Therefore, patients should be monitored closely for signs of toxicity related to a CYP2C8 substrate with a narrow therapeutic index if used concomitantly with abiraterone acetate [see Clinical Pharmacology (12.3) and Warnings and Precautions (5.6)] .
- CYP3A4 Inducers: Avoid concomitant strong CYP3A4 inducers during abiraterone acetate treatment. If a strong CYP3A4 inducer must be co-administered, increase the abiraterone acetate dosing frequency. ( 2.5, 7.1)
- CYP2D6 Substrates: Avoid co-administration of abiraterone acetate with CYP2D6 substrates that have a narrow therapeutic index. If an alternative treatment cannot be used, exercise caution and consider a dose reduction of the concomitant CYP2D6 substrate. ( 7.2)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy and safety of abiraterone acetate with prednisone was established in three randomized placebo-controlled international clinical studies. All patients in these studies received a GnRH analog or had prior bilateral orchiectomy. Patients with prior ketoconazole treatment for prostate cancer and a history of adrenal gland or pituitary disorders were excluded from these trials. Concurrent use of spironolactone was not allowed during the study period.
COU-AA-301: Patients with metastatic CRPC who had received prior docetaxel chemotherapy
In COU-AA-301 (NCT00638690), a total of 1195 patients were randomized 2:1 to receive either abiraterone acetate orally at a dose of 1,000 mg once daily in combination with prednisone 5 mg orally twice daily (N=797) or placebo once daily plus prednisone 5 mg orally twice daily (N=398). Patients randomized to either arm were to continue treatment until disease progression (defined as a 25% increase in PSA over the patient's baseline/nadir together with protocol- defined radiographic progression and symptomatic or clinical progression), initiation of new treatment, unacceptable toxicity or withdrawal.
The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 69 years (range 39 to 95) and the racial distribution was 93% Caucasian, 3.6% Black, 1.7% Asian, and 1.6% Other. Eighty-nine percent of patients enrolled had an ECOG performance status score of 0 to 1 and 45% had a Brief Pain Inventory-Short Form score of ≥4 (patient's reported worst pain over the previous 24 hours). Ninety percent of patients had metastases in bone and 30% had visceral involvement. Seventy percent of patients had radiographic evidence of disease progression and 30% had PSA-only progression. Seventy percent of patients had previously received one cytotoxic chemotherapy regimen and 30% received two regimens.
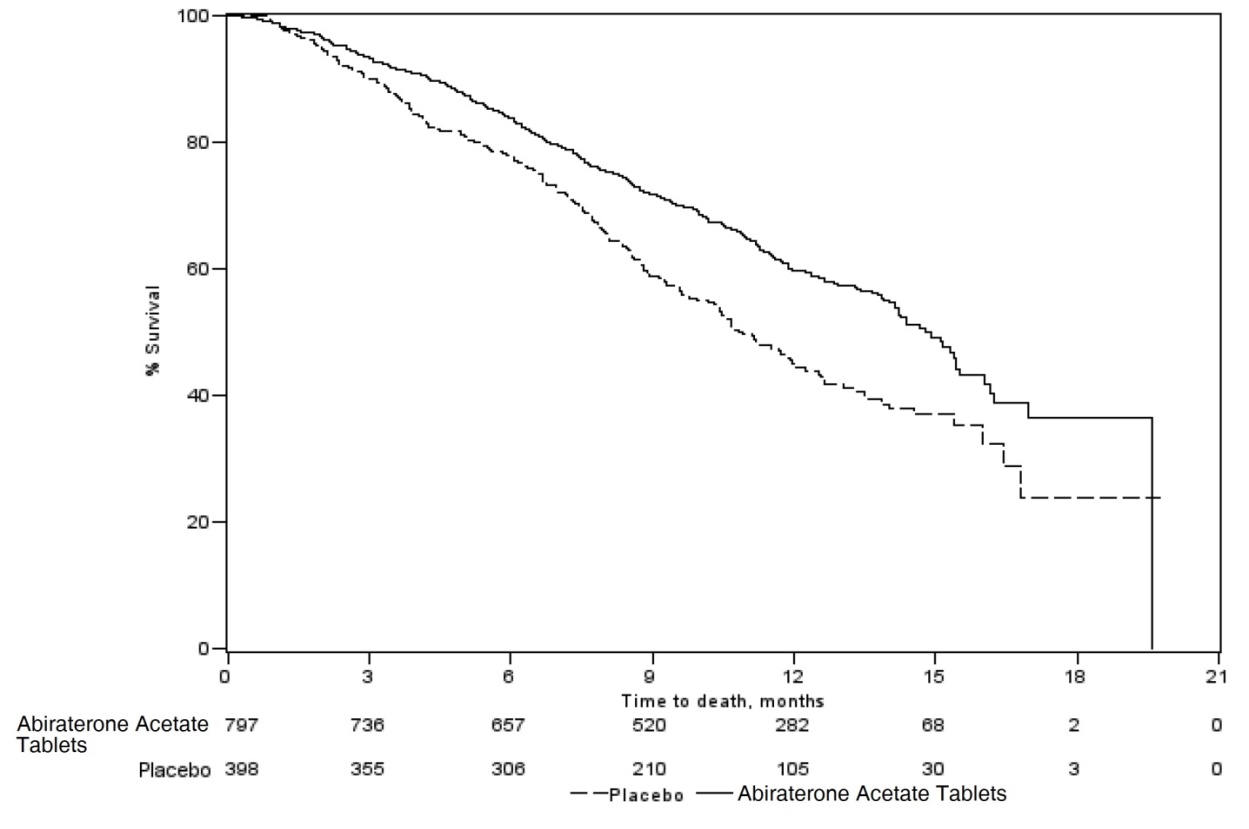
The protocol pre-specified interim analysis was conducted after 552 deaths and showed a statistically significant improvement in overall survival (OS) in patients treated with abiraterone acetate with prednisone compared to patients in the placebo with prednisone arm (Table 9 and Figure 1). An updated survival analysis was conducted when 775 deaths (97% of the planned number of deaths for final analysis) were observed. Results from this analysis were consistent with those from the interim analysis (Table 7).
Table 7: Overall Survival of Patients Treated with Either Abiraterone acetate or Placebo in Combination with Prednisone in COU-AA-301 (Intent-to- Treat Analysis)|
Abiraterone acetate with Prednisone (N=797) |
Placebo with Prednisone (N=398) | |
|---|---|---|
|
Primary Survival Analysis | ||
|
Deaths (%) |
333 (42%) |
219 (55%) |
|
Median survival (months) (95% CI) |
14.8 (14.1, 15.4) |
10.9 (10.2, 12.0) |
|
p-value* |
<0.0001 | |
|
Hazard ratio (95% CI)† |
0.646 (0.543, 0.768) | |
|
Updated Survival Analysis | ||
|
Deaths (%) |
501 (63%) |
274 (69%) |
|
Median survival (months) (95% CI) |
15.8 (14.8, 17.0) |
11.2 (10.4, 13.1) |
|
Hazard ratio (95% CI)† |
0.740 (0.638, 0.859) |
Figure 1: Kaplan-Meier Overall Survival Curves in COU-AA-301 (Intent-to- Treat Analysis)
COU-AA-302: Patients with metastatic CRPC who had not received prior cytotoxic chemotherapy
In COU-AA-302 (NCT00887198), 1088 patients were randomized 1:1 to receive either abiraterone acetate orally at a dose of 1,000 mg once daily (N=546) or Placebo orally once daily (N=542). Both arms were given concomitant prednisone 5 mg twice daily. Patients continued treatment until radiographic or clinical (cytotoxic chemotherapy, radiation or surgical treatment for cancer, pain requiring chronic opioids, or ECOG performance status decline to 3 or more) disease progression, unacceptable toxicity or withdrawal. Patients with moderate or severe pain, opiate use for cancer pain, or visceral organ metastases were excluded.
Patient demographics were balanced between the treatment arms. The median age was 70 years. The racial distribution of patients treated with abiraterone acetate was 95% Caucasian, 2.8% Black, 0.7% Asian and 1.1% Other. The ECOG performance status was 0 for 76% of patients, and 1 for 24% of patients. Co- primary efficacy endpoints were overall survival and radiographic progression- free survival (rPFS). Baseline pain assessment was 0 to 1 (asymptomatic) in 66% of patients and 2 to 3 (mildly symptomatic) in 26% of patients as defined by the Brief Pain Inventory-Short Form (worst pain over the last 24 hours).
Radiographic progression-free survival was assessed with the use of sequential imaging studies and was defined by bone scan identification of 2 or more new bone lesions with confirmation (Prostate Cancer Working Group 2 criteria) and/or modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria for progression of soft tissue lesions. Analysis of rPFS utilized centrally- reviewed radiographic assessment of progression.
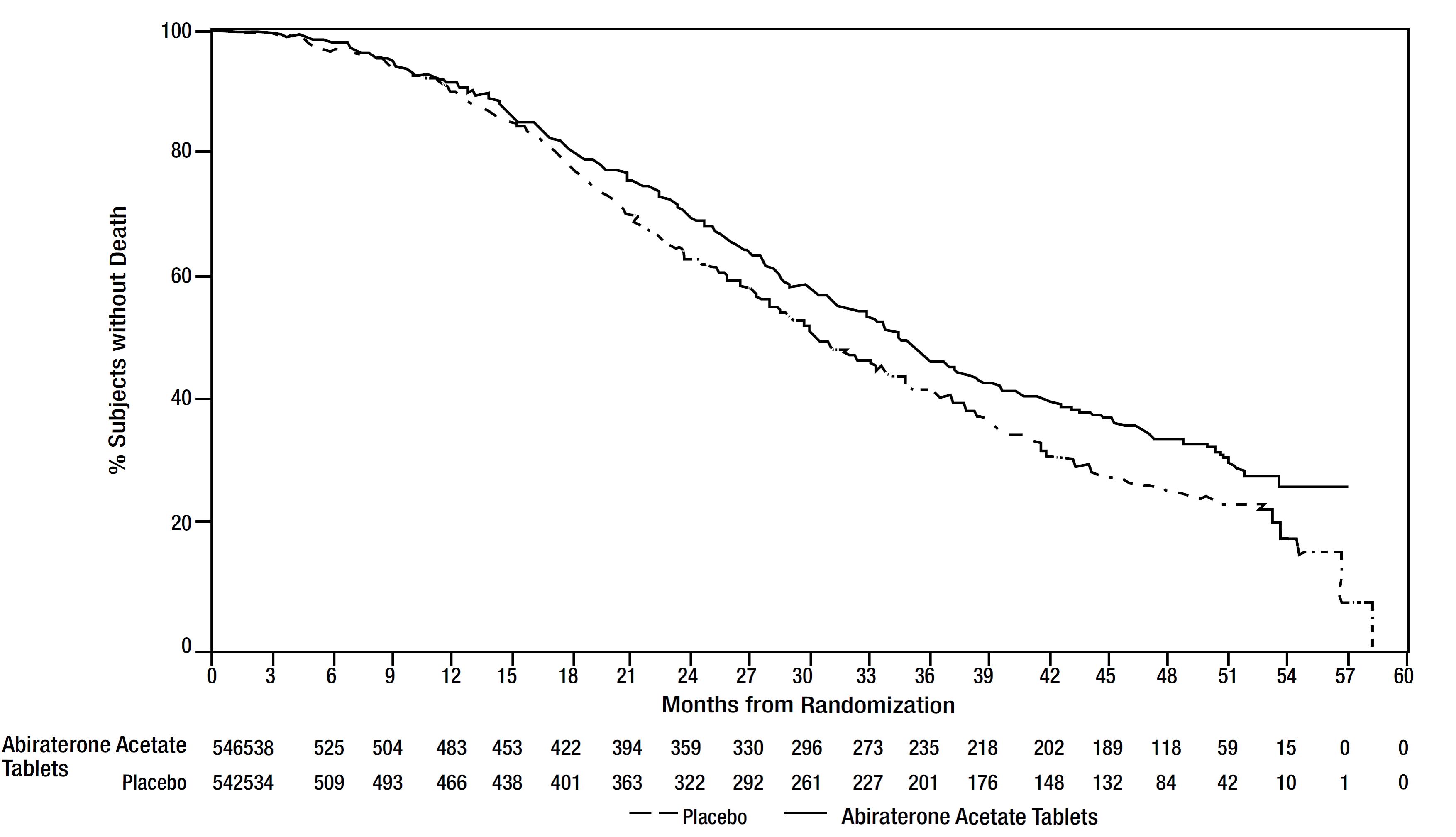
The planned final analysis for OS, conducted after 741 deaths (median follow up of 49 months) demonstrated a statistically significant OS improvement in patients treated with abiraterone acetate with prednisone compared to those treated with placebo with prednisone (Table 8 and Figure 2). Sixty-five percent of patients on the abiraterone acetate arm and 78% of patients on the placebo arm used subsequent therapies that may prolong OS in metastatic CRPC. Abiraterone acetate was used as a subsequent therapy in 13% of patients on the abiraterone acetate arm and 44% of patients on the placebo arm.
Table 8: Overall Survival of Patients Treated with Either Abiraterone acetate or Placebo in Combination with Prednisone in COU-AA-302 (Intent-to- Treat Analysis)|
Abiraterone acetate with Prednisone (N=546) |
Placebo with Prednisone (N=542) | |
|---|---|---|
| ||
|
Overall Survival | ||
|
Deaths |
354 (65%) |
387 (71%) |
|
Median survival (months) (95% CI) |
34.7 (32.7, 36.8) |
30.3 (28.7, 33.3) |
|
p-value * |
0.0033 | |
|
Hazard ratio † (95% CI) |
0.81 (0.70, 0.93) |
Figure 2: Kaplan Meier Overall Survival Curves in COU-AA-302
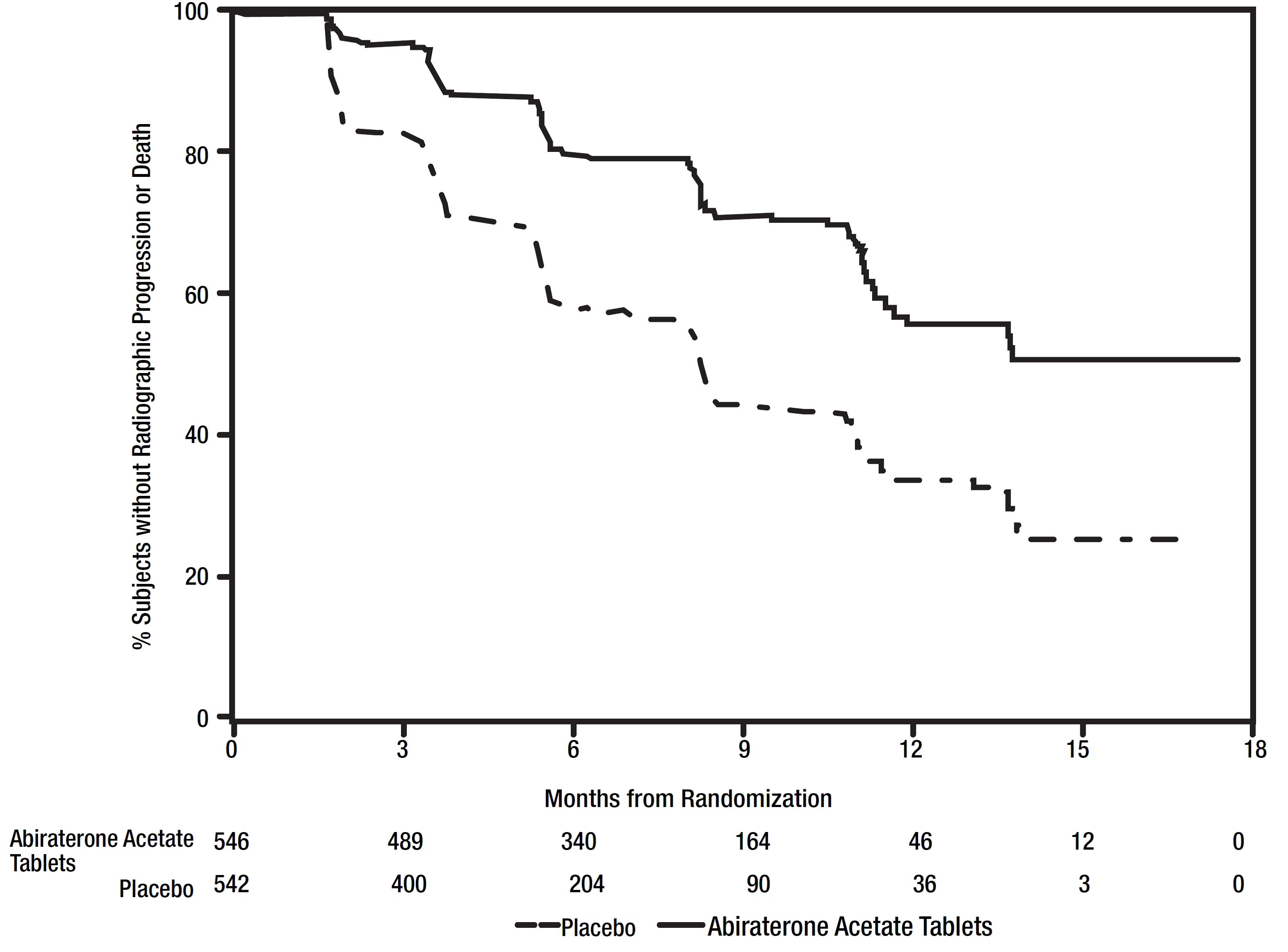
At the pre-specified rPFS analysis, 150 (28%) patients treated with abiraterone acetate with prednisone and 251 (46%) patients treated with placebo with prednisone had radiographic progression. A significant difference in rPFS between treatment groups was observed (Table 9 and Figure 3).
Table 9: Radiographic Progression-free Survival of Patients Treated with Either Abiraterone acetate or Placebo in Combination with Prednisone in COU-AA-302 (Intent-to-Treat Analysis)|
Abiraterone acetate with Prednisone (N=546) |
Placebo with Prednisone (N=542) | |
|---|---|---|
| ||
|
NR=Not reached. | ||
|
Radiographic Progression-free Survival | ||
|
Progression or death |
150 (28%) |
251 (46%) |
|
Median rPFS (months) (95% CI) |
NR (11.66, NR) |
8.28 (8.12, 8.54) |
|
p-value * |
<0.0001 | |
|
Hazard ratio † (95% CI) |
0.425 (0.347, 0.522) |
Figure 3: Kaplan Meier Curves of Radiographic Progression-free Survival in COU-AA-302 (Intent-to-Treat Analysis)
The primary efficacy analyses are supported by the following prospectively defined endpoints. The median time to initiation of cytotoxic chemotherapy was 25.2 months for patients in the abiraterone acetate arm and 16.8 months for patients in the placebo arm (HR=0.580; 95% CI: [0.487, 0.691], p < 0.0001).
The median time to opiate use for prostate cancer pain was not reached for patients receiving abiraterone acetate and was 23.7 months for patients receiving placebo (HR=0.686; 95% CI: [0.566, 0.833], p=0.0001). The time to opiate use result was supported by a delay in patient reported pain progression favoring the abiraterone acetate arm.
LATITUDE: Patients with metastatic high-risk CSPC
In LATITUDE (NCT01715285), 1199 patients with metastatic high-risk CSPC were randomized 1:1 to receive either abiraterone acetate orally at a dose of 1,000 mg once daily with prednisone 5 mg once daily (N=597) or placebo orally once daily (N=602). High-risk disease was defined as having at least two of three risk factors at baseline: a total Gleason score of ≥8, presence of ≥3 lesions on bone scan, and evidence of measurable visceral metastases. Patients with significant cardiac, adrenal, or hepatic dysfunction were excluded. Patients continued treatment until radiographic or clinical disease progression, unacceptable toxicity, withdrawal or death. Clinical progression was defined as the need for cytotoxic chemotherapy, radiation or surgical treatment for cancer, pain requiring chronic opioids, or ECOG performance status decline to ≥3.
Patient demographics were balanced between the treatment arms. The median age was 67 years among all randomized subjects. The racial distribution of patients treated with abiraterone acetate was 69% Caucasian, 2.5% Black, 21% Asian, and 8.1% Other. The ECOG performance status was 0 for 55%, 1 for 42%, and 2 for 3.5% of patients. Baseline pain assessment was 0 to 1 (asymptomatic) in 50% of patients, 2 to 3 (mildly symptomatic) in 23% of patients, and ≥4 in 28% of patients as defined by the Brief Pain Inventory-Short Form (worst pain over the last 24 hours).
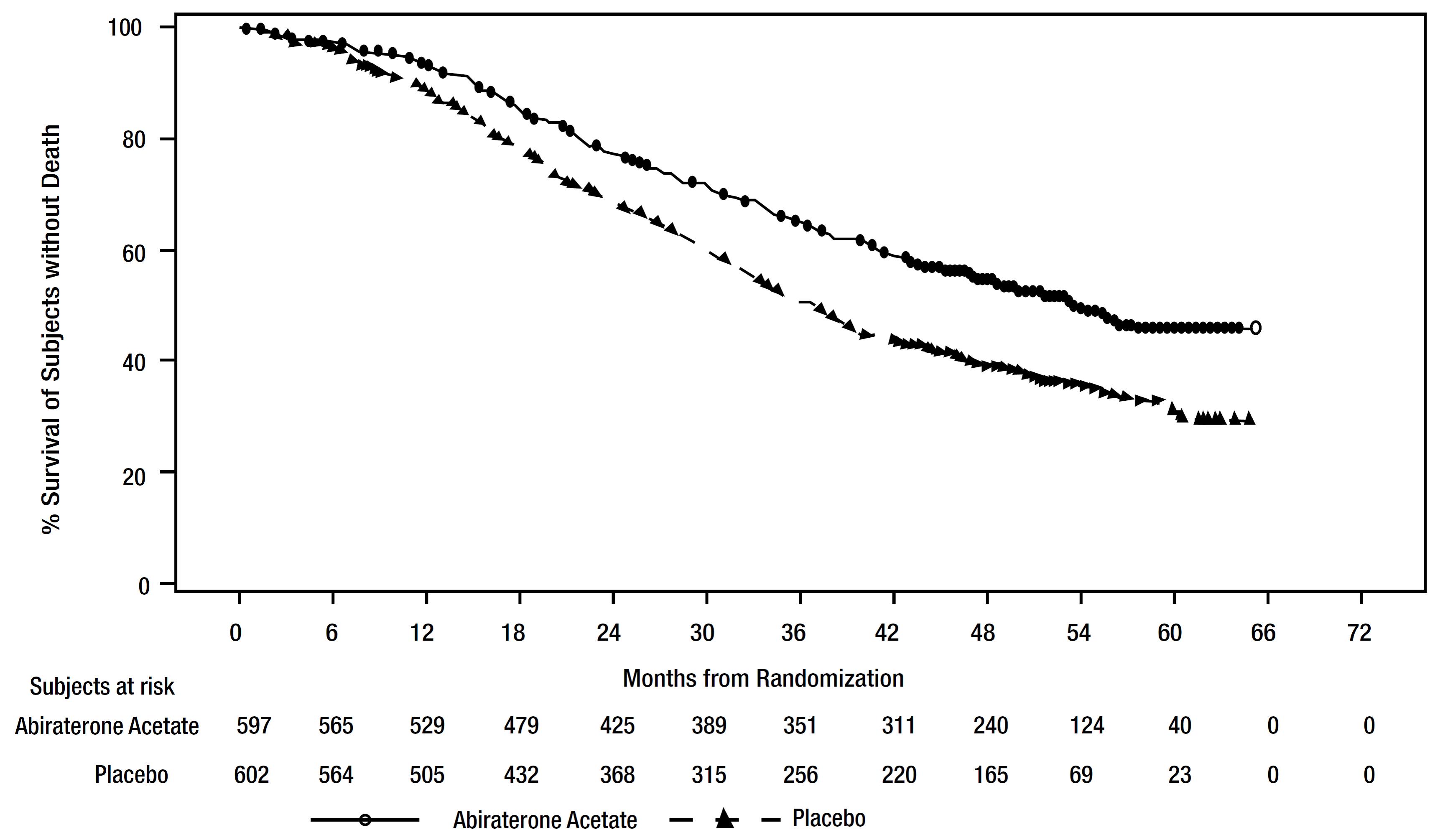
A major efficacy outcome was overall survival. The pre-specified interim analysis after 406 deaths showed a statistically significant improvement in OS in patients on abiraterone acetate with prednisone compared to those on placebos. Twenty-one percent of patients on the abiraterone acetate arm and 41% of patients on the placebos arm received subsequent therapies that may prolong OS in metastatic CRPC. An updated survival analysis was conducted when 618 deaths were observed. The median follow-up time was 52 months. Results from this analysis were consistent with those from the pre-specified interim analysis (Table 10 and Figure 4). At the updated analysis, 29% of patients on the abiraterone acetate arm and 45% of patients on the placebos arm received subsequent therapies that may prolong OS in metastatic CRPC.
Table 10: Overall Survival of Patients Treated with Either Abiraterone acetate or Placebos in LATITUDE (Intent-to-Treat Analysis)|
Abiraterone acetate with Prednisone (N=597) |
Placebos (N=602) | |
|---|---|---|
| ||
|
NE=Not estimable | ||
|
Overall Survival***** | ||
|
Deaths (%) |
169 (28%) |
237 (39%) |
|
Median survival (months) (95% CI) |
NE (NE, NE) |
34.7 (33.1, NE) |
|
p-value † |
<0.0001 | |
|
Hazard ratio (95% CI) ‡ |
0.62 (0.51, 0.76) | |
|
Updated Overall Survival | ||
|
Deaths (%) |
275 (46%) |
343 (57%) |
|
Median survival (months) (95% CI) |
53.3 (48.2, NE) |
36.5 (33.5, 40.0) |
|
Hazard ratio (95% CI) ‡ |
0.66 (0.56, 0.78) |
Figure 4: Kaplan-Meier Plot of Overall Survival; Intent-to-treat Population in LATITUDE Updated Analysis
The major efficacy outcome was supported by a statistically significant delay in time to initiation of chemotherapy for patients in the abiraterone acetate arm compared to those in the placebos arm. The median time to initiation of chemotherapy was not reached for patients on abiraterone acetate with prednisone and was 38.9 months for patients on placebos (HR = 0.44; 95% CI: [0.35, 0.56], p < 0.0001).
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety and efficacy of abiraterone acetate have not been established in females. Based on findings from animal studies and the mechanism of action, abiraterone acetate can cause fetal harm and potential loss of pregnancy.
There are no human data on the use of abiraterone acetate in pregnant women. In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose (see Data).
Data
Animal Data
In an embryo-fetal developmental toxicity study in rats, abiraterone acetate caused developmental toxicity when administered at oral doses of 10, 30 or 100 mg/kg/day throughout the period of organogenesis (gestational days 6 to 17). Findings included embryo-fetal lethality (increased post implantation loss and resorptions and decreased number of live fetuses), fetal developmental delay (skeletal effects) and urogenital effects (bilateral ureter dilation) at doses ≥10 mg/kg/day, decreased fetal ano-genital distance at ≥30 mg/kg/day, and decreased fetal body weight at 100 mg/kg/day. Doses ≥10 mg/kg/day caused maternal toxicity. The doses tested in rats resulted in systemic exposures (AUC) approximately 0.03, 0.1 and 0.3 times, respectively, the AUC in patients.
8.2 Lactation
Risk Summary
The safety and efficacy of abiraterone acetate have not been established in females. There is no information available on the presence of abiraterone acetate in human milk, or on the effects on the breastfed child or milk production.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Based on findings in animal reproduction studies and its mechanism of action, advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 weeks after the final dose of abiraterone acetate [see Use in Specific Populations (8.1)] .
Infertility
Based on animal studies, abiraterone acetate may impair reproductive function and fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of abiraterone acetate in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients receiving abiraterone acetate in randomized clinical trials, 70% of patients were 65 years and over and 27% were 75 years and over. No overall differences in safety or effectiveness were observed between these elderly patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Patients with Hepatic Impairment
The pharmacokinetics of abiraterone were examined in subjects with baseline mild (N=8) or moderate (N=8) hepatic impairment (Child-Pugh Class A and B, respectively) and in 8 healthy control subjects with normal hepatic function. The systemic exposure (AUC) of abiraterone after a single oral 1,000 mg dose of abiraterone acetate increased by approximately 1.1-fold and 3.6-fold in subjects with mild and moderate baseline hepatic impairment, respectively compared to subjects with normal hepatic function.
In another trial, the pharmacokinetics of abiraterone were examined in subjects with baseline severe (N=8) hepatic impairment (Child-Pugh Class C) and in 8 healthy control subjects with normal hepatic function. The systemic exposure (AUC) of abiraterone increased by approximately 7-fold and the fraction of free drug increased 2-fold in subjects with severe baseline hepatic impairment compared to subjects with normal hepatic function.
No dosage adjustment is necessary for patients with baseline mild hepatic impairment. In patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the recommended dose of abiraterone acetate to 250 mg once daily. Do not use abiraterone acetate in patients with baseline severe hepatic impairment (Child-Pugh Class C). If elevations in ALT or AST >5X ULN or total bilirubin >3X ULN occur in patients with baseline moderate hepatic impairment, discontinue abiraterone acetate treatment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)] .
For patients who develop hepatotoxicity during treatment, interruption of treatment and dosage adjustment may be required [see Dosage and Administration (2.4), Warnings and Precautions (5.3), and Clinical Pharmacology (12.3)].
8.7 Patients with Renal Impairment
No dosage adjustment is necessary for patients with renal impairment [see Clinical Pharmacology (12.3)] .
- Do not use abiraterone acetate in patients with baseline severe hepatic impairment (Child-Pugh Class C). ( 8.6)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Abiraterone acetate Tablets, 500 mg are purple colored, oval shaped, biconvex bevel edged film-coated tablets, debossed with “A” one side and “500” on the other side.
Bottles of 60 tablets with child-resistant closure NDC 71921-177-06
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted in the range from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Keep out of reach of children.
Based on its mechanism of action, abiraterone acetate tablets may harm a developing fetus. Women who are pregnant or women who may be pregnant should not handle abiraterone acetate tablets if broken, crushed, or damaged without protection, e.g., gloves [see Use in Specific Populations (8.1)] .
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
- Inform patients that abiraterone acetate is associated with hypertension, hypokalemia, and peripheral edema that may lead to QT prolongation and Torsades de Pointes in patients who develop hypokalemia while taking abiraterone acetate. Advise patients that their blood pressure, serum potassium and signs and symptoms of fluid retention will be monitored clinically at least monthly. Advise patients to adhere to corticosteroids and to report symptoms of hypertension, hypokalemia, or edema to their healthcare provider [see Warnings and Precautions (5.1)] .
Adrenocortical Insufficiency
- Inform patients that abiraterone acetate with prednisone is associated with adrenal insufficiency. Advise patients to report symptoms of adrenocortical insufficiency to their healthcare provider [see Warnings and Precautions (5.2)] .
Hepatotoxicity
- Inform patients that abiraterone acetate is associated with severe hepatotoxicity. Inform patients that their liver function will be monitored using blood tests. Advise patients to immediately report symptoms of hepatotoxicity to their healthcare provider [see Warnings and Precautions (5.3)] .
Hypoglycemia
- Inform patients that severe hypoglycemia has been reported when abiraterone acetate was administered to patients with pre-existing diabetes who were receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide – antidiabetic drugs. Advise patients with diabetes to monitor glucose levels during and after treatment with abiraterone acetate [see Warnings and Precautions (5.6) and Drug Interactions (7.2)].
Use in Combination with Radium Ra 223 Dichloride
- Advise patients that radium Ra 223 dichloride showed an increase in mortality and an increased rate of fracture when used in combination with abiraterone acetate plus prednisone/prednisolone. Inform patients to speak with their healthcare provider about any other medications or treatment they are currently taking for prostate cancer [ see Warnings and Precautions (5.4)].
Dosing and Administration
- Inform patients that abiraterone acetate tablets are taken once daily with prednisone (once or twice daily according to their healthcare provider's instructions) and to not interrupt or stop either of these medications without consulting their healthcare provider.
- Inform patients receiving GnRH therapy that they need to maintain this treatment during the course of treatment with abiraterone acetate tablets.
- Instruct patients to take abiraterone acetate tablets as a single dose once daily on an empty stomach. Instruct patients to not eat food 2 hous before and 1 hour after taking Abiraterone acetate tablets. Abiraterone acetate tablets taken with food causes increased exposure and may result in adverse reactions. Instruct patients to swallow tablets whole with water and not to crush or chew the tablets [see Dosage and Administration (2.3)] .
- Inform patients that if they miss a dose of abiraterone acetate tablets or prednisone, they should take their normal dose the following day. If more than one daily dose is skipped, inform patients to contact their healthcare provider [see Dosage and Administration (2.3)] .
Embryo-Fetal Toxicity
- Inform patients that abiraterone acetate may harm a developing fetus and can cause loss of pregnancy.
- Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 weeks after the final dose of abiraterone acetate tablets [see Use in Specific Populations (8.1)] .
- Advise females who are pregnant or women who may be pregnant not to handle abiraterone acetate tablets if broken, crushed, or damaged without protection, e.g., gloves [see Use in Specific Populations (8.1) and How Supplied/Storage and Handling (16)] .
Infertility
- Advise male patients that abiraterone acetate may impair fertility [see Use in Specific Populations (8.3)] .
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose for Metastatic CRPC
The recommended dose of abiraterone acetate tablets is 1,000 mg (two 500 mg tablets or four 250 mg tablets) orally once daily with prednisone 5 mg orally twice daily.
2.2 Recommended Dose for Metastatic High-risk CSPC
The recommended dose of abiraterone acetate tablets is 1,000 mg (two 500 mg tablets or four 250 mg tablets) orally once daily with prednisone 5 mg administered orallyonce daily.
2.3 Important Administration Instructions
Patients receiving abiraterone acetate tablets should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy.
Abiraterone acetate tablets must be taken as a single dose once daily on an empty stomach. Do not eat food 2 hours before and 1 hour after taking abiraterone acetate tablets. The tablets must be swallowed whole with water. Do not crush or chew tablets.
2.4 Dose Modification Guidelines in Hepatic Impairment and Hepatotoxicity
Hepatic Impairment
In patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the recommended dose of abiraterone acetate tablets to 250 mg once daily. In patients with moderate hepatic impairment monitor ALT, AST, and bilirubin prior to the start of treatment, every week for the first month, every two weeks for the following two months of treatment and monthly thereafter. If elevations in ALT and/or AST greater than 5X d upper limit of normal (ULN) or total bilirubin greater than 3X ULN occur in patients with baseline moderate hepatic impairment, discontinue abiraterone acetate tablets and do not re-treat patients with abiraterone acetate tablets [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] .
Do not use abiraterone acetate tablets in patients with baseline severe hepatic impairment (Child-Pugh Class C).
Hepatotoxicity
For patients who develop hepatotoxicity during treatment with abiraterone acetate tablets (ALT and/or AST greater than 5X ULN or total bilirubin greater than 3X ULN), interrupt treatment with abiraterone acetate tablets [see Warnings and Precautions (5.3)]. Treatment may be restarted at a reduced dose of 750 mg once daily following return of liver function tests to the patient's baseline or to AST and ALT less than or equal to 2.5X ULN and total bilirubin less than or equal to 1.5X ULN . For patients who resume treatment, monitor serum transaminases and bilirubin at a minimum of every two weeks for three months and monthly thereafter.
If hepatotoxicity recurs at the dose of 750 mg once daily, re-treatment may be restarted at a reduced dose of 500 mg once daily following return of liver function tests to the patient's baseline or to AST and ALT less than or equal to 2.5× ULN and total bilirubin less than or equal to 1.5X ULN .
If hepatotoxicity recurs at the reduced dose of 500 mg once daily, discontinue treatment with abiraterone acetate tablets.
Permanently discontinue abiraterone acetate tablets for patients who develop a concurrent elevation of ALT greater than 3 × ULN and total bilirubin greater than 2 × ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation [see Warnings and Precautions (5.3)] .
2.5 Dose Modification Guidelines for Strong CYP3A4 Inducers
Avoid concomitant strong CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital) during abiraterone acetate tablets treatment.
If a strong CYP3A4 inducer must be co-administered, increase the abiraterone acetate tablets dosing frequency to twice a day only during the co- administration period (e.g., from 1,000 mg once daily to 1,000 mg twice a day). Reduce the dose back to the previous dose and frequency, if the concomitant strong CYP3A4 inducer is discontinued [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)] .
Metastatic castration-resistant prostate cancer:
- Abiraterone acetate tablets 1,000 mg orally once daily with prednisone 5 mg orallytwice daily. ( 2.1)
Metastatic castration-sensitive prostate cancer:
- Abiraterone acetate tablets 1,000 mg orally once daily with prednisone 5 mg orallyoncedaily. ( 2.2)
Patients receiving abiraterone acetate tablets should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. Abiraterone acetate tablets must be taken as a single dose once daily on an empty stomach. Do not eat food 2 hours before and 1 hour after taking Abiraterone acetate tablets. The tablets must be swallowed whole with water. Do not crush or chew tablets. ( 2.3)
Dose Modification:
- For patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the abiraterone acetate tablets starting dose to 250 mg once daily. ( 2.4)
- For patients who develop hepatotoxicity during treatment, hold abiraterone acetate tablets until recovery. Retreatment may be initiated at a reduced dose. Abiraterone acetate tablets should be discontinued if patients develop severe hepatotoxicity. ( 2.4)
