ofloxacin
Ofloxacin Ophthalmic Solution USP, 0.3% sterile
871d63f4-41c1-4b30-903c-11fbf0d4c1f8
HUMAN PRESCRIPTION DRUG LABEL
Aug 31, 2023
Lifestar Pharma LLC
DUNS: 080268943
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ofloxacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
ofloxacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
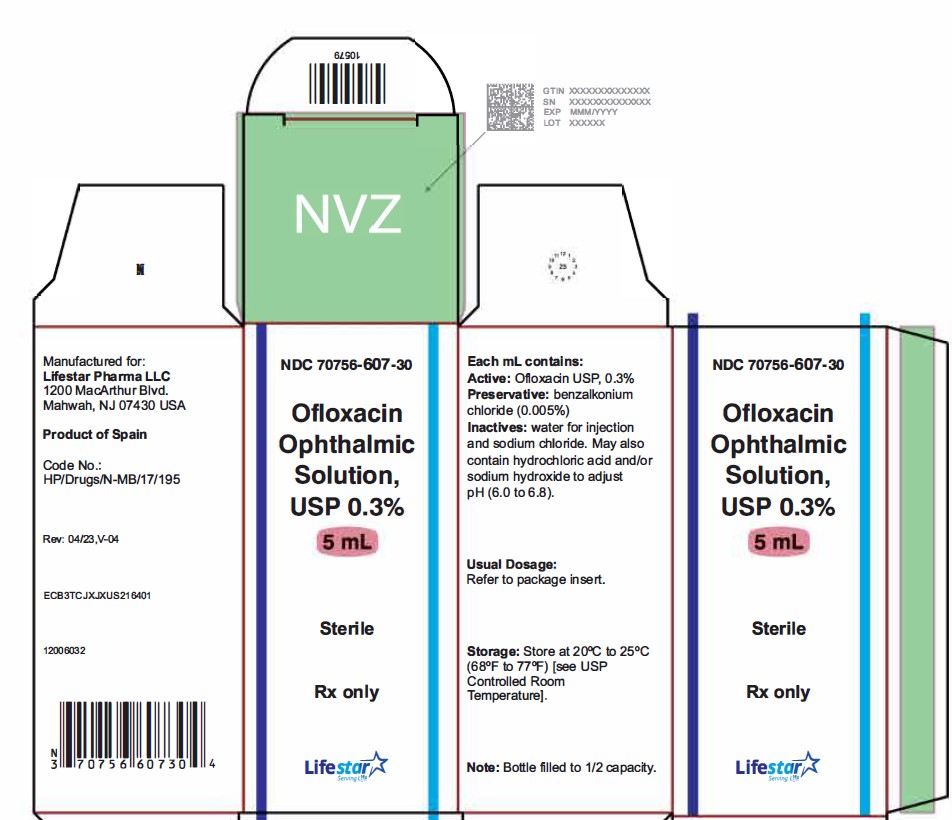
NDC 70756-607-30
Ofloxacin Ophthalmic
Solution, USP 0.3%
Sterile
Rx only
5 mL

NDC 70756-607-30
Ofloxacin Ophthalmic
Solution, USP 0.3%
5 mL
Sterile
Rx only
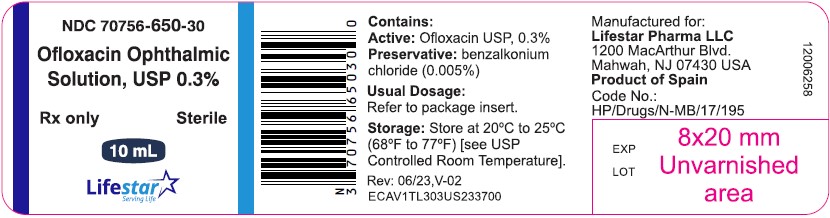
NDC 70756-650-30
Ofloxacin Ophthalmic
Solution, USP 0.3%
Sterile
Rx only
10 mL
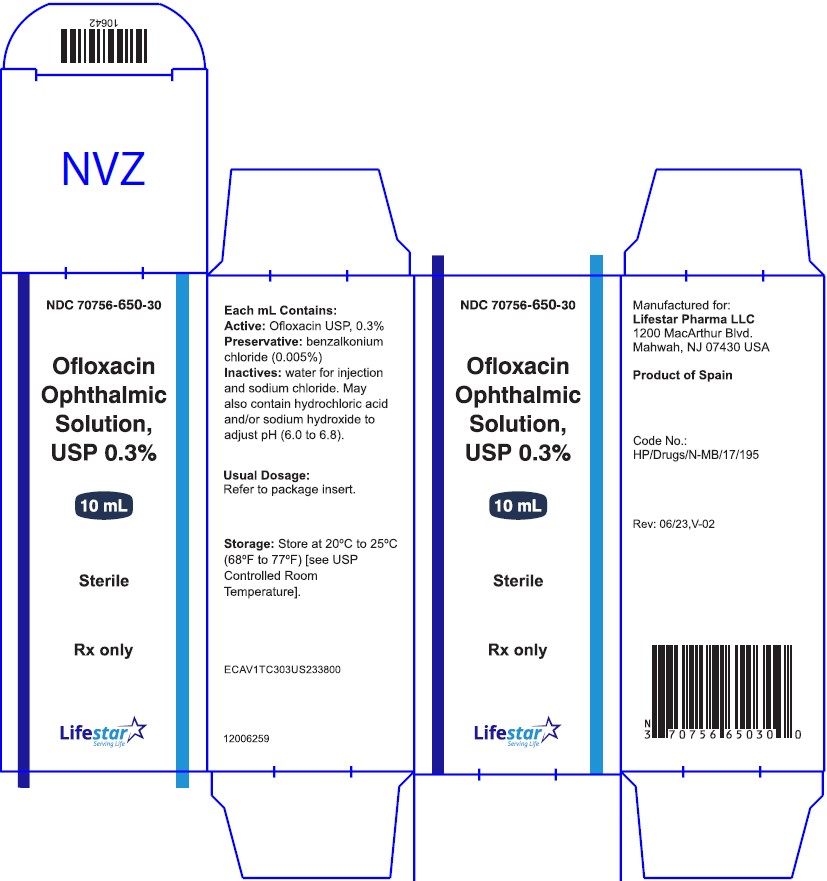
NDC 70756-650-30
Ofloxacin Ophthalmic
Solution, USP 0.3%
Sterile
Rx only
10 mL
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Ophthalmic Use
The most frequently reported drug-related adverse reaction was transient ocular burning or discomfort. Other reported reactions include stinging, redness, itching, chemical conjunctivitis/keratitis, ocular/periocular/facial edema, foreign body sensation, photophobia, blurred vision, tearing, dryness, and eye pain. Rare reports of dizziness and nausea have been received.
Refer to Warnings for additional adverse reactions.
To report SUSPECTED ADVERSE REACTIONS, contact Lifestar Pharma LLC at 1-888-995-4337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
DESCRIPTION
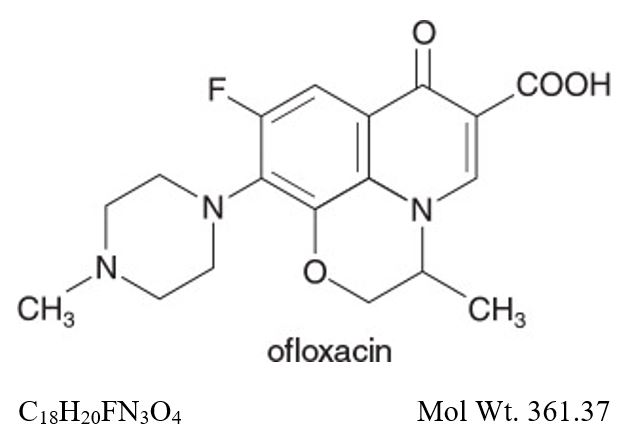
**Ofloxacin ophthalmic solution USP, 0.3%**is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical ophthalmic use.
Chemical Name:
(±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid.
**Contains: Active:**ofloxacin 0.3% (3 mg/mL).**Preservative:**benzalkonium chloride (0.005%).
**Inactives:**sodium chloride and water for injection. May also contain hydrochloric acid and/or sodium hydroxide to adjust pH.
Ofloxacin ophthalmic solution, USP is unbuffered and formulated with a pH of 6.4 (range - 6.0 to 6.8). It has an osmolality of 300 mOsm/kg. Ofloxacin is a fluorinated 4-quinolone which differs from other fluorinated 4-quinolones in that there is a six member (pyridobenzoxazine) ring from positions 1 to 8 of the basic ring structure.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
The recommended dosage regimen for the treatment of**bacterial conjunctivitis **is:
|
Days 1 and 2 |
Instill one to two drops every two to four hours in the affected eye(s). |
|
Days 3 through 7 |
Instill one to two drops four times daily. |
|
The recommended dosage regimen for the treatment of** bacterial corneal ulcer** is: | |
|
Days 1 and 2 |
Instill one to two drops into the affected eye every 30 minutes, while awake. Awaken at approximately four and six hours after retiring and instill one to two drops. |
|
Days 3 through 7 to 9 |
Instill one to two drops hourly, while awake. |
|
Days 7 to 9 through | |
|
treatment completion |
Instill one to two drops, four times daily. |
