DRISDOL
Drisdol
Approved
Approval ID
acb61678-d23d-405d-8c14-1a16cfd3a776
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Mar 14, 2023
Manufacturers
FDA
Validus Pharmaceuticals LLC
DUNS: 801194619
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ergocalciferol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code30698-493
Application NumberNDA003444
Product Classification
M
Marketing Category
C73594
G
Generic Name
ergocalciferol
Product Specifications
Route of AdministrationORAL
Effective DateMay 8, 2023
FDA Product Classification
INGREDIENTS (6)
ERGOCALCIFEROLActive
Quantity: 1.25 mg in 1 1
Code: VS041H42XC
Classification: ACTIB
GELATINInactive
Code: 2G86QN327L
Classification: IACT
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
FD&C YELLOW NO. 5Inactive
Code: I753WB2F1M
Classification: IACT
FD&C BLUE NO. 1Inactive
Code: H3R47K3TBD
Classification: IACT
SOYBEAN OILInactive
Code: 241ATL177A
Classification: IACT
Drug Labeling Information
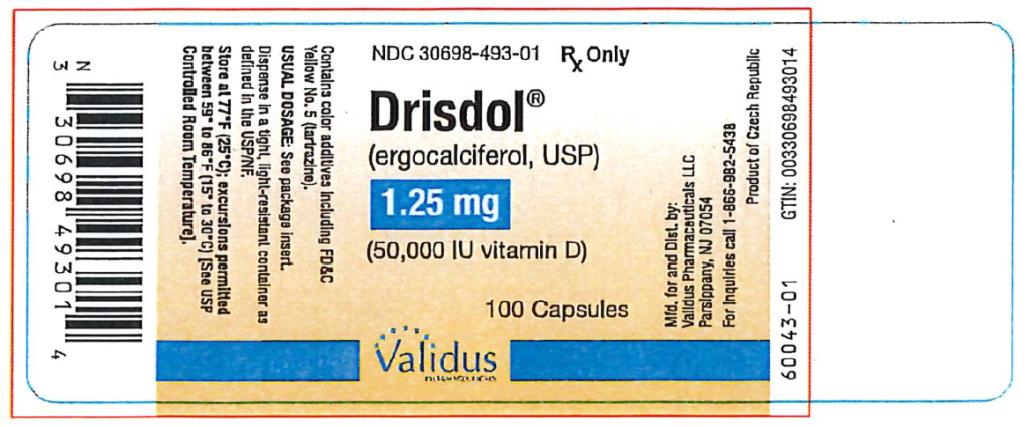
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 3/14/2023
PRINCIPAL DISPLAY PANEL
NDC 30698-493-01
Drisdol
(ergocalciferol, USP)
1.25
(50,000 IU vitamin D)
100 Capsules
Rx Only