Atazanavir Sulfate
These highlights do not include all the information needed to use ATAZANAVIR CAPSULES safely and effectively. See full prescribing information for ATAZANAVIR CAPSULES. ATAZANAVIR capsules, for oral use Initial U.S. Approval: 2003
0e152eab-74fb-413f-914d-5b005b58f847
HUMAN PRESCRIPTION DRUG LABEL
Feb 16, 2024
NorthStar Rx LLC
DUNS: 830546433
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Atazanavir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Atazanavir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Atazanavir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (30 Capsules Bottle)
Rx only
NDC 16714-862-01
Atazanavir Capsules
300 mg
ALERT: Find out about medicinesthat should
** NOT be taken withAtazanavir Capsules.**
NORTHSTAR**X™**** 30 Capsules**
****
Indications & Usage Section
1 INDICATIONS AND USAGE
Atazanavir capsule is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and in pediatric patients 6 years and older weighing at least 15 kg.
Limitations of Use:
- Atazanavir capsules are not recommended for use in pediatric patients below the age of 3 months due to the risk of kernicterus [see Use in Specific Populations (8.4)].
- Use of atazanavir capsules with ritonavir in treatment-experienced patients should be guided by the number of baseline primary protease inhibitor resistance substitutions [see Microbiology (12.4)].
Atazanavir capsule is a protease inhibitor indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and in pediatric patients 6 years and older weighing at least 15 kg. (1)
Contraindications Section
4 CONTRAINDICATIONS
Atazanavir capsules are contraindicated:
- in patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of atazanavir capsules [see Warnings and Precautions (5.2)].
- when coadministered with drugs that are highly dependent on CYP3A or UGT1A1 for clearance, and for which elevated plasma concentrations of the interacting drugs are associated with serious and/or life-threatening events (see Table 6).
- when coadministered with drugs that strongly induce CYP3A and may lead to lower exposure and loss of efficacy of atazanavir capsules (see Table 6).
Table 6 displays drugs that are contraindicated with atazanavir capsules.
Table 6: Drugs Contraindicated with Atazanavir Capsules (Information in the table applies to atazanavir capsules with or without ritonavir, unless otherwise indicated)|
a See Drug Interactions, Table 16 (7) for parenterally administered midazolam. | |
|
Drug Class |
Drugs within class that are contraindicated with atazanavir capsules |
|
Alpha 1-adrenoreceptor antagonist |
Alfuzosin |
|
Antiarrhythmics |
Amiodarone (with ritonavir), quinidine (with ritonavir) |
|
Anticonvulsants |
Carbamazepine, phenobarbital, phenytoin |
|
Antimycobacterials |
Rifampin |
|
Antineoplastics |
Apalutamide, encorafenib, irinotecan, ivosidenib |
|
Antipsychotics |
Lurasidone (with ritonavir), pimozide |
|
Benzodiazepines |
Orally administered midazolama, triazolam |
|
Ergot Derivatives |
Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
|
GI Motility Agent |
Cisapride |
|
Hepatitis C Direct-Acting Antivirals |
Elbasvir/grazoprevir; glecaprevir/pibrentasvir |
|
Herbal Products |
St. John’s wort (Hypericum perforatum) |
|
Lipid-Modifying Agents: |
Lomitapide, lovastatin, simvastatin |
|
Phosphodiesterase-5 (PDE-5) Inhibitor |
Sildenafilb when dosed as REVATIO® for the treatment of pulmonary arterial hypertension |
|
Protease Inhibitors |
Indinavir |
|
Non-nucleoside Reverse Transcriptase Inhibitors |
Nevirapine |
- Atazanavir capsules are contraindicated in patients with previously demonstrated hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product. (4)
- Coadministration with alfuzosin, amiodarone (if atazanavir capsules are coadministered with ritonavir), carbamazepine, quinidine (if atazanavir capsules are coadministered with ritonavir), triazolam, orally administered midazolam, ergot derivatives, rifampin, apalutamide, encorafenib, irinotecan, ivosidenib, lurasidone (if atazanavir is coadministered with ritonavir), lovastatin, simvastatin, lomitapide, indinavir, cisapride, phenobarbital, phenytoin, pimozide, St. John's wort, nevirapine, elbasvir/grazoprevir, glecaprevir/pibrentasvir, and sildenafil when dosed as REVATIO®. (4)
Adverse Reactions Section
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- cardiac conduction abnormalities [see Warnings and Precautions (5.1)]
- rash [see Warnings and Precautions (5.2)]
- hyperbilirubinemia [see Warnings and Precautions (5.8)]
- chronic kidney disease [see Warnings and Precautions (5.5)]
- nephrolithiasis and cholelithiasis [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Treatment-Naive Adult Subjects
The safety profile of atazanavir in treatment-naive adults is based on 1625 subjects with HIV-1 infection in clinical trials. 536 subjects received atazanavir 300 mg with ritonavir 100 mg and 1089 subjects received atazanavir 400 mg or higher (without ritonavir).
The most common adverse reactions were nausea, jaundice/scleral icterus, and rash.
Selected clinical adverse reactions of moderate or severe intensity reported in ≥ 2% of treatment-naive subjects receiving combination therapy including atazanavir 300 mg with ritonavir 100 mg and atazanavir 400 mg (without ritonavir) are presented in Tables 7 and 8, respectively.
Table 7: Selected Adverse Reactionsa of Moderate or Severe Intensity Reported in ≥2% of Adult Treatment-Naive Subjects with HIV-1 Infection,b Study AI424-138|
96 weeks****c |
96 weeks****c | |
|
Digestive System | ||
|
Nausea |
4% |
8% |
|
Jaundice/scleral icterus |
5% |
|
|
Diarrhea |
2% |
12% |
|
Skin and Appendages | ||
|
Rash |
3% |
2% |
|
|
Study AI424-034 |
Studies AI424-007, -008 | |||
|
64 weeksc atazanavir |
64 weeksc efavirenz 600 mg (once daily) with lamivudine/ |
120 weeksc,d atazanavir 400 mg (once daily) with stavudine and
lamivudine or didanosine |
73 weeksc,d nelfinavir | |
|
Body as a Whole | ||||
|
Headache |
6% |
6% |
1% |
2% |
|
Digestive System | ||||
|
Nausea |
14% |
12% |
6% |
4% |
|
Jaundice/scleral icterus |
7% |
|
7% |
|
|
Vomiting |
4% |
7% |
3% |
3% |
|
Abdominal pain |
4% |
4% |
4% |
2% |
|
Diarrhea |
1% |
2% |
3% |
16% |
|
Nervous System | ||||
|
Insomnia |
3% |
3% |
<1% |
|
|
Dizziness |
2% |
7% |
<1% |
|
|
Peripheral neurologic symptoms |
<1% |
1% |
4% |
3% |
|
Skin and Appendages | ||||
|
Rash |
7% |
10% |
5% |
1% |
|
Adverse Reactions in Treatment-Experienced Adult Subjects
The safety profile of atazanavir in treatment-experienced adults with HIV-1 infection is based on 119 subjects with HIV-1 infection in clinical trials.
The most common adverse reactions are jaundice/scleral icterus and myalgia.
Selected clinical adverse reactions of moderate or severe intensity reported in ≥2% of treatment-experienced subjects receiving atazanavir with ritonavir are presented in Table 9.
Table 9: Selected Adverse Reactionsa of Moderate or Severe Intensity Reported in ≥2% of Adult Treatment-Experienced Subjects with HIV-1 Infection,b Study AI424-045|
48 weeks****c |
48 weeks****c | |
|
Body as a Whole | ||
|
Fever |
2% |
|
|
Digestive System | ||
|
Jaundice/scleral icterus |
9% |
|
|
Diarrhea |
3% |
11% |
|
Nausea |
3% |
2% |
|
Nervous System | ||
|
Depression |
2% |
<1% |
|
Musculoskeletal System | ||
|
Myalgia |
4% |
|
|
Laboratory Abnormalities in Treatment-Naive Subjects
The percentages of adult treatment-naive subjects with HIV-1 infection treated with combination therapy, including atazanavir 300 mg with ritonavir 100 mg or atazanavir 400 mg (without ritonavir) with Grade 3 to 4 laboratory abnormalities, are presented in Tables 10 and 11, respectively.
Table 10: Grade 3 to 4 Laboratory Abnormalities Reported in ≥2% of Adult Treatment-Naive Subjects with HIV-1 Infection,a Study AI424-138|
Variable |
Limit****e |
96 weeks****b |
96 weeks****b |
|
Chemistry |
High | ||
|
SGOT/AST |
≥5.1 x ULN |
3% |
1% |
|
SGPT/ALT |
≥5.1 x ULN |
3% |
2% |
|
Total Bilirubin |
≥2.6 x ULN |
44% |
<1% |
|
Lipase |
≥2.1 x ULN |
2% |
2% |
|
Creatine Kinase |
≥5.1 x ULN |
8% |
7% |
|
Total Cholesterol |
≥240 mg/dL |
11% |
25% |
|
Hematology |
Low | ||
|
Neutrophils |
<750 cells/mm3 |
5% |
2% |
|
a Based on the regimen containing atazanavir. |
|
Variable |
Limit****d |
Study AI424-034 |
Studies AI424-007, -008 | ||
|
64 weeks****b |
64 weeks****b |
120 weeks****b,c |
73 weeks****b,c (n=191) | ||
|
Chemistry |
High | ||||
|
SGOT/AST |
≥5.1 x ULN |
2% |
2% |
7% |
5% |
|
SGPT/ALT |
≥5.1 x ULN |
4% |
3% |
9% |
7% |
|
Total Bilirubin |
≥2.6 x ULN |
35% |
<1% |
47% |
3% |
|
Amylase |
≥2.1 x ULN |
|
|
14% |
10% |
|
Lipase |
≥2.1 x ULN |
<1% |
1% |
4% |
5% |
|
Creatine Kinase |
≥5.1 x ULN |
6% |
6% |
11% |
9% |
|
Total Cholesterol |
≥240 mg/dL |
6% |
24% |
19% |
48% |
|
Triglycerides |
≥751 mg/dL |
<1% |
3% |
4% |
2% |
|
Hematology |
Low | ||||
|
Hemoglobin |
<8.0 g/dL |
5% |
3% |
<1% |
4% |
|
Neutrophils |
<750 cells/mm3 |
7% |
9% |
3% |
7% |
|
Change in Lipids from Baseline in Treatment-Naive Subjects with HIV-1 Infection
For Study AI424-138 and Study AI424-034, changes from baseline in LDL- cholesterol, HDL- cholesterol, total cholesterol, and triglycerides are shown in Tables 12 and 13, respectively.
Table 12: Lipid Values, Mean Change from Baseline, Study AI424-138|
atazanavir with ritonavir****a,b |
lopinavir/ritonavir****b,c | |||||||||
|
Baseline |
Week 48 |
Week 96 |
Baseline |
Week 48 |
Week 96 | |||||
|
mg/dL |
mg/dL |
Change****d |
mg/dL |
Change****d |
mg/dL |
mg/dL |
Change****d |
mg/dL |
Change****d | |
|
LDL-Cholesterolf |
92 |
105 |
+14% |
105 |
+14% |
93 |
111 |
+19% |
110 |
+17% |
|
HDL-Cholesterolf |
37 |
46 |
+29% |
44 |
+21% |
36 |
48 |
+37% |
46 |
+29% |
|
Total Cholesterolf |
149 |
169 |
+13% |
169 |
+13% |
150 |
187 |
+25% |
186 |
+25% |
|
Triglyceridesf |
126 |
145 |
+15% |
140 |
+13% |
129 |
194 |
+52% |
184 |
+50% |
|
a Atazanavir 300 mg with ritonavir 100 mg once daily with the fixed-dose
product: 300 mg tenofovir DF/200 mg emtricitabine once daily. |
|
atazanavir****a,b |
efavirenz****b,c | |||||
|
Baseline |
Week 48 |
Week 48 |
Baseline |
Week 48 |
Week 48 | |
|
mg/dL |
mg/dL |
Change****d |
mg/dL |
mg/dL |
Change****d | |
|
LDL-Cholesterolf |
98 |
98 |
+1% |
98 |
114 |
+18% |
|
HDL-Cholesterol |
39 |
43 |
+13% |
38 |
46 |
+24% |
|
Total Cholesterol |
164 |
168 |
+2% |
162 |
195 |
+21% |
|
Triglyceridesf |
138 |
124 |
-9% |
129 |
168 |
+23% |
|
a Atazanavir 400 mg once daily with the fixed-dose product: 150 mg lamivudine,
300 mg zidovudine twice daily. |
Laboratory Abnormalities in Treatment-Experienced Subjects with HIV-1 Infection
The percentages of adult treatment-experienced subjects with HIV-1 infection treated with combination therapy, including atazanavir with ritonavir having Grade 3 to 4 laboratory abnormalities, are presented in Table 14.
Table 14: Grade 3 to 4 Laboratory Abnormalities Reported in ≥2% of Adult Treatment-Experienced Subjects with HIV-1 Infection, Study AI424-045a|
Variable |
Limit****c |
48 weeks****b |
48 weeksblopinavir/ritonavir |
|
Chemistry |
High | ||
|
SGOT/AST |
≥5.1 x ULN |
3% |
3% |
|
SGPT/ALT |
≥5.1 x ULN |
4% |
3% |
|
Total Bilirubin |
≥2.6 x ULN |
49% |
<1% |
|
Lipase |
≥2.1 x ULN |
5% |
6% |
|
Creatine Kinase |
≥5.1 x ULN |
8% |
8% |
|
Total Cholesterol |
≥240 mg/dL |
25% |
26% |
|
Triglycerides |
≥751 mg/dL |
8% |
12% |
|
Glucose |
≥251 mg/dL |
5% |
<1% |
|
Hematology |
Low | ||
|
Platelets |
<50,000 cells/mm3 |
2% |
3% |
|
Neutrophils |
<750 cells/mm3 |
7% |
8% |
|
a Based on regimen(s) containing atazanavir. |
Change in Lipids from Baseline in Treatment-Experienced Subjects with HIV-1 Infection
For Study AI424-045, changes from baseline in LDL-cholesterol, HDL- cholesterol, total cholesterol, and triglycerides are shown in Table 15. The observed magnitude of dyslipidemia was less with atazanavir with ritonavir than with lopinavir/ritonavir. However, the clinical impact of such findings has not been demonstrated.
Table 15: Lipid Values, Mean Change from Baseline, Study AI424-045|
Atazanavir** with ritonavir****a,b** |
Lopinavir/ritonavir****b,c | |||||
|
Baseline |
Week 48 |
Week 48 |
Baseline |
Week 48 |
Week 48 | |
|
mg/dL |
mg/dL |
Change****d |
mg/dL |
mg/dL |
Change****d | |
|
LDL-Cholesterolf |
108 |
98 |
-10% |
104 |
103 |
+1% |
|
HDL-Cholesterol |
40 |
39 |
-7% |
39 |
41 |
+2% |
|
Total Cholesterol |
188 |
170 |
-8% |
181 |
187 |
+6% |
|
Triglyceridesf |
215 |
161 |
-4% |
196 |
224 |
+30% |
|
a Atazanavir 300 mg once daily with ritonavir and tenofovir DF, and 1 NRTI. |
Adverse Reactions in Pediatric Subjects with HIV-1 Infection: Atazanavir Capsules
The safety and tolerability of atazanavir capsules with and without ritonavir have been established in pediatric subjects with HIV-1 infection, at least 6 years of age from the open-label, multicenter clinical trial PACTG 1020A.
The safety profile of atazanavir in pediatric subjects with HIV-1 infection (6 to less than 18 years of age) taking the capsule formulation was generally similar to that observed in clinical studies of atazanavir in adults. The most common Grade 2 to 4 adverse events (≥5%, regardless of causality) reported in pediatric subjects were cough (21%), fever (18%), jaundice/scleral icterus (15%), rash (14%), vomiting (12%), diarrhea (9%), headache (8%), peripheral edema (7%), extremity pain (6%), nasal congestion (6%), oropharyngeal pain (6%), wheezing (6%), and rhinorrhea (6%). Asymptomatic second-degree atrioventricular block was reported in <2% of subjects. The most common Grade 3 to 4 laboratory abnormalities occurring in pediatric subjects taking the capsule formulation were elevation of total bilirubin (≥3.2 mg/dL, 58%), neutropenia (9%), and hypoglycemia (4%). All other Grade 3 to 4 laboratory abnormalities occurred with a frequency of less than 3%.
Adverse Reactions in Subjects with HIV-1 Infection, Co-Infected with Hepatitis B and/or Hepatitis C Virus
In Study AI424-138, 60 subjects administered atazanavir 300 mg with ritonavir 100 mg once daily, and 51 subjects treated with lopinavir/ritonavir 400 mg/100 mg (as fixed-dose product) twice daily, each with fixed-dose tenofovir DF/emtricitabine, were seropositive for hepatitis B and/or C at study entry. ALT levels >5 times ULN developed in 10% (6/60) of the subjects administered atazanavir with ritonavir and 8% (4/50) of the subjects treated with lopinavir/ritonavir. AST levels >5 times ULN developed in 10% (6/60) of the subjects administered atazanavir with ritonavir and none (0/50) of the subjects treated with lopinavir/ritonavir.
In Study AI424-045, 20 subjects administered atazanavir 300 mg with ritonavir 100 mg once daily, and 18 subjects treated with lopinavir/ritonavir 400 mg/100 mg twice daily (as fixed-dose product), were seropositive for hepatitis B and/or C at study entry. ALT levels >5 times ULN developed in 25% (5/20) of the subjects administered atazanavir with ritonavir and 6% (1/18) of the subjects treated with lopinavir/ritonavir treated. AST levels >5 times ULN developed in 10% (2/20) of the subjects administered atazanavir with ritonavir and 6% (1/18) of the subjects treated with lopinavir/ritonavir.
In Studies AI424-008 and AI424-034, 74 subjects treated with atazanavir 400 mg once daily, 58 who received efavirenz, and 12 who received nelfinavir were seropositive for hepatitis B and/or C at study entry. ALT levels >5 times ULN developed in 15% of the subjects treated with atazanavir, 14% of the subjects treated with efavirenz, and 17% of the subjects treated with nelfinavir. AST levels >5 times ULN developed in 9% of the subjects treated with atazanavir, 5% of the subjects treated with efavirenz, and 17% of the subjects treated with nelfinavir. Within atazanavir and control regimens, no difference in frequency of bilirubin elevations was noted between seropositive and seronegative subjects [see Warnings and Precautions (5.8)].
6.2 Postmarketing Experience
The following events have been identified during postmarketing use of atazanavir. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: edema
Cardiovascular System: second-degree AV block, third-degree AV block, left bundle branch block, QTc prolongation [see Warnings and Precautions (5.1)]
Gastrointestinal System: pancreatitis
Hepatic System: hepatic function abnormalities
Hepatobiliary Disorders: cholelithiasis [see Warnings and Precautions (5.6)], cholecystitis, cholestasis
Metabolic System and Nutrition Disorders: diabetes mellitus, hyperglycemia [see Warnings and Precautions (5.9)]
Musculoskeletal System: arthralgia
Renal System: nephrolithiasis [see Warnings and Precautions (5.6)], interstitial nephritis, granulomatous interstitial nephritis, chronic kidney disease [see Warnings and Precautions (5.5)]
Skin and Appendages: alopecia, maculopapular rash [see Contraindications (4) and Warnings and Precautions (5.2)], pruritus, angioedema
Most common adverse reactions (≥2%) are nausea, jaundice/scleral icterus, rash, headache, abdominal pain, vomiting, insomnia, peripheral neurologic symptoms, dizziness, myalgia, diarrhea, depression, and fever. (6.1)
** To report SUSPECTED ADVERSE REACTIONS, contact Northstar Rx LLC at 1-800-206-7821 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.**
Drug Interactions Section
7 DRUG INTERACTIONS
7.1 Potential for Atazanavir to Affect Other Drugs
Atazanavir is an inhibitor of CYP3A and UGT1A1. Coadministration of atazanavir and drugs primarily metabolized by CYP3A or UGT1A1 may result in increased plasma concentrations of the other drug that could increase or prolong its therapeutic and adverse effects.
Atazanavir is a weak inhibitor of CYP2C8. Use of atazanavir without ritonavir is not recommended when coadministered with drugs highly dependent on CYP2C8 with narrow therapeutic indices (e.g., paclitaxel, repaglinide). When atazanavir with ritonavir is coadministered with substrates of CYP2C8, clinically significant interactions are not expected [see Clinical Pharmacology, Table 22 (12.3)].
The magnitude of CYP3A-mediated drug interactions on coadministered drug may change when atazanavir is coadministered with ritonavir. See the complete prescribing information for ritonavir for information on drug interactions with ritonavir.
7.2 Potential for Other Drugs to Affect Atazanavir
Atazanavir is a CYP3A4 substrate; therefore, drugs that induce CYP3A4 may decrease atazanavir plasma concentrations and reduce atazanavir's therapeutic effect.
Atazanavir solubility decreases as pH increases. Reduced plasma concentrations of atazanavir are expected if proton-pump inhibitors, antacids, buffered medications, or H2-receptor antagonists are administered with atazanavir [see Dosage and Administration (2.3, 2.4, and 2.6)].
7.3 Established and Other Potentially Significant Drug Interactions
Table 16 provides dosing recommendations in adults as a result of drug interactions with atazanavir. These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious events or loss of efficacy.
Table 16: Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studiesa or Predicted Interactions (Information in the table applies to atazanavir with or without ritonavir, unless otherwise indicated)|
Concomitant Drug Class: |
Effect on Concentration |
Clinical Comment |
|
HIV Antiviral Agents | ||
|
Nucleoside Reverse Transcriptase Inhibitors (NRTIs): |
↓ atazanavir |
It is recommended that atazanavir be given (with food) 2 h before or 1 h after didanosine buffered formulations. Simultaneous administration of didanosine EC and atazanavir with food results in a decrease in didanosine exposure. Thus, atazanavir and didanosine EC should be administered at different times. |
|
Nucleotide Reverse Transcriptase Inhibitors: |
↓ atazanavir |
When coadministered with tenofovir DF in adults, it is recommended that atazanavir 300 mg be given with ritonavir 100 mg and tenofovir DF 300 mg (all as a single daily dose with food). The mechanism of this interaction is unknown. Higher tenofovir concentrations could potentiate tenofovir-associated adverse reactions, including renal disorders. Patients receiving atazanavir and tenofovir DF should be monitored for tenofovir- associated adverse reactions. For pregnant patients taking atazanavir with ritonavir and tenofovir DF, see Dosage and Administration (2.6). |
|
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs): efavirenz |
↓ atazanavir |
In HIV-treatment-naive adult patients: In HIV-treatment-experienced adult patients: |
|
nevirapine |
↓ atazanavir |
Coadministration of atazanavir with nevirapine is contraindicated due to the potential loss of virologic response and development of resistance, as well as the potential risk for nevirapine-associated adverse reactions [see Contraindications (4)]. |
|
Protease Inhibitors: |
↑ saquinavir |
Appropriate dosing recommendations for this combination, with or without ritonavir, with respect to efficacy and safety have not been established. In a clinical study, saquinavir 1200 mg coadministered with atazanavir 400 mg and tenofovir DF 300 mg (all given once daily), and nucleoside analogue reverse transcriptase inhibitors did not provide adequate efficacy [see Clinical Studies (14.2)]. |
|
indinavir |
Coadministration of atazanavir with indinavir is contraindicated. Both atazanavir and indinavir are associated with indirect (unconjugated) hyperbilirubinemia [see Contraindications (4)]. | |
|
ritonavir |
↑ atazanavir |
If atazanavir is coadministered with ritonavir, it is recommended that atazanavir 300 mg once daily be given with ritonavir 100 mg once daily with food in adults. See the complete prescribing information for ritonavir for information on drug interactions with ritonavir. |
|
Others |
↑ other protease inhibitor |
Coadministration with other protease inhibitors is not recommended. |
|
Hepatitis C Antiviral Agents | ||
|
elbasvir/grazoprevir |
↑ grazoprevir |
Coadministration of atazanavir with grazoprevir is contraindicated due to the potential for increased risk of ALT elevations [see Contraindications (4)]. |
|
glecaprevir/pibrentasvir |
↑ glecaprevir |
Coadministration of atazanavir with glecaprevir/pibrentasvir is contraindicated due to the potential for increased the risk of ALT elevations [see Contraindications (4)]. |
|
voxilaprevir/sofosbuvir/velpatasvir |
↑ voxilaprevir |
Coadministration with atazanavir is not recommended. |
|
Other Agents | ||
|
Alpha 1-Adrenoreceptor Antagonist: alfuzosin |
↑ alfuzosin |
Coadministration of atazanavir with alfuzosin is contraindicated due to risk for hypotension [see Contraindications (4)]. |
|
Antacids and buffered medications: |
↓ atazanavir |
Atazanavir should be administered 2 hours before or 1 hour after antacids and buffered medications. |
|
Antiarrhythmics: amiodarone, quinidine amiodarone, bepridil, lidocaine (systemic), quinidine |
↑ amiodarone, bepridil, lidocaine (systemic), quinidine |
Concomitant use of atazanavir with ritonavir and either quinidine or amiodarone is contraindicated due to the potential for serious or life- threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. Coadministration with atazanavir without ritonavir has the potential to produce serious and/or life-threatening adverse events but has not been studied. Caution is warranted and therapeutic concentration monitoring of these drugs is recommended if they are used concomitantly with atazanavir without ritonavir. |
|
Anticoagulants: warfarin |
↑ warfarin |
Coadministration with atazanavir has the potential to produce serious and/or life-threatening bleeding and has not been studied. It is recommended that International Normalized Ratio (INR) be monitored. |
|
Direct-Acting Oral Anticoagulants: betrixaban, dabigatran, edoxaban |
↑ betrixaban |
Concomitant use of atazanavir with ritonavir, a strong CYP3A4/P-gp inhibitor, may result in an increased risk of bleeding. Refer to the respective DOAC prescribing information regarding dosing instructions for coadministration with P-gp inhibitors. |
|
rivaroxaban |
Atazanavir with ritonavir Atazanavir Atazanavir with ritonavir Atazanavir |
Coadministration of atazanavir with ritonavir, a strong CYP3A4/P-gp inhibitor,
and rivaroxaban is not recommended, as it may result in an Coadministration of atazanavir, a CYP3A4 inhibitor, and rivaroxaban may result in an increased risk of bleeding. Close monitoring is recommended when atazanavir is coadministered with rivaroxaban. Concomitant use of atazanavir with ritonavir, a strong CYP3A4/P-gp inhibitor, may result in an increased risk of bleeding. Refer to apixaban dosing instructions for coadministration with strong CYP3A4 and P-gp inhibitors in the apixaban prescribing information. Concomitant use of atazanavir, a CYP3A4 inhibitor, and apixaban may result in an increased risk of bleeding. Close monitoring is recommended when apixaban is coadministered with atazanavir. |
|
Antidepressants: tricyclic antidepressants |
↑ tricyclic |
Coadministration with atazanavir has the potential to produce serious and/or life-threatening adverse events and has not been studied. Concentration monitoring of these drugs is recommended if they are used concomitantly with atazanavir. |
|
trazodone |
↑ trazodone |
Nausea, dizziness, hypotension, and syncope have been observed following coadministration of trazodone with ritonavir. If trazodone is used with a CYP3A4 inhibitor such as atazanavir, the combination should be used with caution and a lower dose of trazodone should be considered. |
|
Antiepileptics: |
↓ atazanavir |
Coadministration of atazanavir (with or without ritonavir) with carbamazepine is contraindicated due to the risk for loss of virologic response and development of resistance [see Contraindications (4)]. |
|
phenytoin, phenobarbital |
↓ atazanavir |
Coadministration of atazanavir (with or without ritonavir) with phenytoin or |
|
lamotrigine |
↓ lamotrigine |
Coadministration of lamotrigine and atazanavirwith ritonavir may require
dosage adjustment of lamotrigine. |
|
Antifungals: |
Atazanavir with ritonavir: |
Coadministration of ketoconazole has only been studied with atazanavir without ritonavir (negligible increase in atazanavir AUC and Cmax). Due to the effect of ritonavir on ketoconazole, high doses of ketoconazole and itraconazole (>200 mg/day) should be used cautiously when administering atazanavir with ritonavir. |
|
voriconazole |
Atazanavir with ritonavir Atazanavir with ritonavir |
The use of voriconazole in patients receiving atazanavir with ritonavir is not recommended unless an assessment of the benefit/risk to the patient justifies the use of voriconazole. Patients should be carefully monitored for voriconazole-associated adverse reactions and loss of either voriconazole or atazanavir efficacy during the coadministration of voriconazole and atazanavir with ritonavir. Coadministration of voriconazole with atazanavir (without ritonavir) may affect atazanavir concentrations; however, no data are available. |
|
Antigout: colchicine |
↑ colchicine |
The coadministration of atazanavir with colchicine in patients with renal or hepatic impairment is not recommended. **Recommended adult dosage of colchicine when administered with
**atazanavir: Prophylaxis of gout flares: If the original regimen was 0.6 mg once a day, the regimen should be adjusted
to 0.3 mg once every other day. |
|
Antimycobacterials: rifampin |
↓ atazanavir |
Coadministration of atazanavir with rifampin is contraindicated due to the risk for loss of virologic response and development of resistance [see Contraindications (4)]. |
|
rifabutin |
↑ rifabutin |
A rifabutin dose reduction of up to 75% (e.g., 150 mg every other day or 3 times per week) is recommended. Increased monitoring for rifabutin-associated adverse reactions including neutropenia is warranted. |
|
Antineoplastics: irinotecan apalutamide ivosidenib encorafenib |
↑ irinotecan ↓ atazanavir ↓ atazanavir ↓ atazanavir |
Coadministration of atazanavir with irinotecan is contraindicated. Atazanavir
inhibits UGT1A1 and may interfere with the metabolism of irinotecan, resulting
in increased irinotecan toxicities [see Contraindications (4)]. Coadministration of ivosidenib with atazanavir (with or without ritonavir) is contraindicated due to the potential for loss of virologic response and risk of serious adverse events such as QT interval prolongation. Coadministration of encorafenib with atazanavir (with or without ritonavir) is contraindicated due to the potential for the loss of virologic response and risk of serious adverse events such as QT interval prolongation. |
|
Antiplatelets clopidogrel |
↑ ticagrelor ↓ clopidogrel active metabolite |
Coadministration with ticagrelor is not recommended due to potential increase in the risk of dyspnea, bleeding and other adverse events associated with ticagrelor. Coadministration of atazanavir (with or without ritonavir) and clopidogrel is not recommended. This is due to the potential reduction of the antiplatelet activity of clopidogrel. |
|
Antipsychotics: |
↑ pimozide |
Coadministration of atazanavir with pimozide is contraindicated. This is due to the potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)] |
|
lurasidone |
Atazanavir with ritonavir Atazanavir ↑ quetiapine |
Atazanavir with ritonavir Atazanavir without ritonavir Initiation of atazanavir with ritonavir in patients taking quetiapine: Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking atazanavir with ritonavir: |
|
Benzodiazepines: |
↑ midazolam |
Coadministration of atazanavir with either orally administered midazolam or triazolam is contraindicated. Triazolam and orally administered midazolam are extensively metabolized by CYP3A4, and coadministration with atazanavir can lead to the potential for serious and/or life-threatening events such as prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
|
parenterally administered |
↑ midazolam |
Coadministration with parenteral midazolam should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. |
|
Calcium channel blockers: diltiazem |
↑ diltiazem and |
Caution is warranted. A dose reduction of diltiazem by 50% should be considered. ECG monitoring is recommended. Coadministration of diltiazem and atazanavir with ritonavir has not been studied. |
|
felodipine, nifedipine, nicardipine, and verapamil |
↑ calcium |
Caution is warranted. Dose titration of the calcium channel blocker should be considered. ECG monitoring is recommended. |
|
Corticosteroids: |
↓ atazanavir |
Coadministration with dexamethasone or other corticosteroids that induce CYP3A may result in loss of therapeutic effect of atazanavir and development of resistance to atazanavir and/or ritonavir. Alternative corticosteroids should be considered. Coadministration with corticosteroids (all routes of administration) that are metabolized by CYP3A, particularly for long-term use, may increase the risk for development of systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression. Consider the potential benefit of treatment versus the risk of systemic corticosteroid effects. For coadministration of cutaneously administered corticosteroids sensitive to CYP3A inhibition, refer to the prescribing information of the corticosteroid for additional information. |
|
Endothelin receptor antagonists: |
Atazanavir Atazanavir with ritonavir ↑ bosentan |
Coadministration of bosentan and atazanavir without ritonavir is not recommended. For adult patients who have been receiving atazanavir with ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based on individual tolerability. For adult patients who have been receiving bosentan, discontinue bosentan at least 36 hours before starting atazanavir with ritonavir. At least 10 days after starting atazanavir with ritonavir, resume bosentan at 62.5 mg once daily or every other day based on individual tolerability. |
|
Ergot derivatives: dihydroergotamine, ergotamine, ergonovine, methylergonovine |
↑ ergot derivatives |
Coadministration of atazanavir with ergot derivatives is contraindicated. This is due to the potential for serious and/or life-threatening events such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [see Contraindications (4)]. |
|
GI Motility Agents: cisapride |
↑ cisapride |
Coadministration of atazanavir with cisapride is contraindicated. This is due to the potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
|
Gonadotropin-releasing hormone Receptor (GnRH) |
↓ atazanavir |
Coadministration of elagolix and atazanavir with or without ritonavir is not recommended due to the potential of loss of virologic response and the potential risk of adverse events such as bone loss and hepatic transaminase elevations associated with elagolix. In the event coadministration is necessary, limit concomitant use of elagolix 200 mg twice daily with atazanavir with or without ritonavir for up to 1 month or limit concomitant use of elagolix 150 mg once daily with atazanavir (with or without ritonavir) for up to 6 months and monitor virologic response. |
|
Herbal Products: St. John’s wort (Hypericum perforatum) |
↓ atazanavir |
Coadministration of products containing St. John’s wort with atazanavir is contraindicated. This may result in loss of therapeutic effect of atazanavir and the development of resistance [see Contraindications (4)]. |
|
Kinase inhibitors: |
↑ R406 (active metabolite |
When coadministering fostamatinib with atazanavir (with or without ritonavir), monitor for toxicities of R406 exposure resulting in dose-related adverse events such as hepatotoxicity and neutropenia. Fostamatinib dose reduction may be required. |
|
Lipid-modifying agents |
↑ lovastatin |
Coadministration of atazanavir with lovastatin or simvastatin is contraindicated. This is due to the potential for serious reactions such as myopathy, including rhabdomyolysis [see Contraindications (4)]. |
|
atorvastatin, rosuvastatin |
↑ atorvastatin |
Titrate atorvastatin dose carefully and use the lowest necessary dose. Rosuvastatin dose should not exceed 10 mg/day. The risk of myopathy, including rhabdomyolysis, may be increased when HIV protease inhibitors, including atazanavir, are used in combination with these drugs. |
|
Other Lipid Modifying Agents: lomitapide |
↑ lomitapide |
Coadministration of atazanavir with lomitapide is contraindicated. This is due to the potential for risk of markedly increased transaminase levels and hepatotoxicity associated with increased plasma concentrations of lomitapide. The mechanism of interaction is CYP3A4 inhibition by atazanavir and/or ritonavir [see Contraindications (4)]. |
|
H2-Receptor antagonists |
↓ atazanavir |
Coadministration may result in loss of virologic response and development of resistance. In HIV-treatment-naive adult patients: OR For patients unable to tolerate ritonavir, atazanavir 400 mg once daily with food should be administered at least 2 hours before and at least 10 hours after a dose of the H2RA. No single dose of the H2RA should exceed a dose comparable to famotidine 20 mg, and the total daily dose should not exceed a dose comparable to famotidine 40 mg. The use of atazanavir without ritonavir in pregnant patients is not recommended. In treatment-experienced adult patients: |
|
Hormonal contraceptives: ethinyl estradiol and norgestimate or norethindrone |
↓ ethinyl estradiol ↑ norgestimatec ↑ ethinyl estradiol ↑ norethindroned |
Use caution if considering coadministration of oral contraceptives with atazanavir or atazanavir with ritonavir. If atazanavir with ritonavir is coadministered with an oral contraceptive, it is recommended that the oral contraceptive contain at least 35 mcg of ethinyl estradiol. If atazanavir is administered without ritonavir, the oral contraceptive should contain no more than 30 mcg of ethinyl estradiol. Potential safety risks include substantial increases in progesterone exposure. The long-term effects of increases in concentration of the progestational agent are unknown and could increase the risk of insulin resistance, dyslipidemia, and acne. Coadministration of atazanavir or atazanavir with ritonavir and other hormonal contraceptives (e.g., contraceptive patch, contraceptive vaginal ring, or injectable contraceptives) or oral contraceptives containing progestogens other than norethindrone or norgestimate, or less than 25 mcg of ethinyl estradiol, has not been studied; therefore, alternative methods of contraception are recommended. |
|
Immunosuppressants: cyclosporine, sirolimus, tacrolimus |
↑ immunosuppressants |
Therapeutic concentration monitoring is recommended for these immunosuppressants when coadministered with atazanavir. |
|
Inhaled beta agonist: salmeterol |
↑ salmeterol |
Coadministration of salmeterol with atazanavir is not recommended. Concomitant use of salmeterol and atazanavir may result in increased risk of cardiovascular adverse reactions associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
|
Inhaled/nasal steroid: fluticasone |
Atazanavir |
Concomitant use of fluticasone propionate and atazanavir without ritonavir should be used with caution. Consider alternatives to fluticasone propionate, particularly for long-term use. |
|
Atazanavir****with ritonavir |
With concomitant use of fluticasone propionate and atazanavir with ritonavir systemic corticosteroid effects, including Cushing’s syndrome and adrenal suppression, have been reported during postmarketing use in patients receiving ritonavir and inhaled or intranasally administered fluticasone propionate. Coadministration of fluticasone propionate and atazanavir with ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects [see Warnings and Precautions (5.1)]. | |
|
Macrolide antibiotics: clarithromycin |
↑ clarithromycin |
Increased concentrations of clarithromycin may cause QTc prolongations; therefore, a dose reduction of clarithromycin by 50% should be considered when it is coadministered with atazanavir. In addition, concentrations of the active metabolite 14-OH clarithromycin are significantly reduced; consider alternative therapy for indications other than infections due to Mycobacterium avium complex. Coadministration of atazanavir with ritonavir and clarithromycin has not been studied. |
|
Opioids: buprenorphine |
Atazanavir or atazanavir****with ritonavir Atazanavir |
Coadministration of atazanavir with ritonavir and buprenorphine warrants clinical monitoring for sedation and cognitive effects. A dose reduction of buprenorphine may be considered. The coadministration of atazanavir and buprenorphine without ritonavir is not recommended. |
|
PDE5 inhibitors: sildenafil, tadalafil, vardenafil |
↑ sildenafil |
Coadministration with atazanavir has not been studied but may result in an increase in PDE5 inhibitor-associated adverse reactions, including hypotension, syncope, visual disturbances, and priapism. Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH): The following dose adjustments are recommended for the use of ADCIRCA® (tadalafil) with atazanavir: Coadministration of ADCIRCA® in patients on atazanavir (with or without ritonavir):
Coadministration of atazanavir (with or without ritonavir) in patients on ADCIRCA®:
Use of PDE5 inhibitors for erectile dysfunction: Use CIALIS® (tadalafil) with caution at reduced doses of 10 mg every 72 hours with increased monitoring for adverse events. **Atazanavir with ritonavir:**Use vardenafil with caution at reduced doses of no more than 2.5 mg every 72 hours with increased monitoring for adverse reactions. **Atazanavir:**Use vardenafil with caution at reduced doses of no more than 2.5 mg every 24 hours with increased monitoring for adverse reactions. |
|
Proton-pump inhibitors: omeprazole |
↓ atazanavir |
Coadministration of atazanavir with or without ritonavir and omeprazole may result in loss of virologic response and development of resistance. In HIV-treatment-naive adult patients: In HIV-treatment-experienced adult patients: |
|
a For magnitude of interactions see Clinical Pharmacology, Tables 21 and 22
(12.3). |
7.4 Drugs with No Observed Interactions with Atazanavir
No clinically significant drug interactions were observed when atazanavir was coadministered with methadone, fluconazole, acetaminophen, atenolol, or the nucleoside reverse transcriptase inhibitors lamivudine or zidovudine [see Clinical Pharmacology, Tables 21 and 22 (12.3)].
Coadministration of atazanavir can alter the concentration of other drugs and other drugs may alter the concentration of atazanavir. The potential drug-drug interactions must be considered prior to and during therapy. (4, 7, 12.3)
Recent Major Changes Section
- None reported in this treatment arm.
a Includes events of possible, probable, certain, or unknown relationship to treatment regimen.
b Based on the regimen containing atazanavir.
c Median time on therapy.
d Administered as a fixed-dose
e As a fixed-dose product: 300 mg tenofovir DF, 200 mg emtricitabine once daily.
Dosage Forms & Strengths Section
3 DOSAGE FORMS AND STRENGTHS
- 150 mg Capsules are blue/powder blue size ‘1’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘150 mg’ on blue cap and ‘T24’ on powder blue body with white edible ink.
- 200 mg Capsules are blue/blue size ‘0’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘200 mg’ on blue cap and ‘T25’ on blue body with white edible ink.
- 300 mg Capsules are red/blue size ‘00’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘300 mg’ on red cap and ‘T26’ on blue body with white edible ink.
- Capsules: 150 mg, 200 mg, and 300 mg. (3, 16)
How Supplied Section
16 HOW SUPPLIED/STORAGE AND HANDLING
Atazanavir Capsules, 150 mgare blue/powder blue size ‘1’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘150 mg’ on blue cap and ‘T24’ on powder blue body with white edible ink.
Bottles of 60 NDC 16714-860-01
** Atazanavir Capsules, 200 mg**are blue/blue size ‘0’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘200 mg’ on blue cap and ‘T25’ on blue body with white edible ink.
Bottles of 60 NDC 16714-861-01
** Atazanavir Capsules, 300 mg**are red/blue size ‘00’ hard gelatin capsule filled with off-white to pale yellow granular powder and imprinted with ‘300 mg’ on red cap and ‘T26’ on blue body with white edible ink.
Bottles of 30 NDC 16714-862-01
Keep capsules in a tightly closed container.
Store at 20o to 25oC (68o to 77oF) [see USP Controlled Room Temperature].
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
Atazanavir Capsules
(A-ta-ZAN-a-vir)
**Important: Ask your healthcare provider or pharmacist about medicines that should not be taken withatazanavir capsules. For more information, see “Donot takeatazanavir capsules if you”**and “Before taking atazanavir capsules”.
What are atazanavir capsules**?**
Atazanavir capsules are a prescription medicine that is used to treat human immunodeficiency virus-1 (HIV-1) infection, in combination with other HIV-1 medicines in adults and children at least 6 years of age and older and weighing at least 15 kg.
HIV-1 is the virus that causes AIDS (Acquired Immunodeficiency Syndrome).
Atazanavir capsules should not be used in children younger than 3 months of age.
Do not take atazanavir capsules if you:
- are allergic to atazanavir or any of the ingredients in atazanavir capsules. See the end of this leaflet for a complete list of ingredients in atazanavir capsules.
- are taking any of the following medicines. Taking atazanavir capsules with these medicines may affect how atazanavir capsules work. Atazanavir capsules may cause serious or life-threatening side effects, or death when used with these medicines:
-
alfuzosin
-
amiodarone (when atazanavir capsules are used with ritonavir)
-
apalutamide
-
carbamazepine
-
cisapride
-
elbasvir and grazoprevir
-
encorafenib
-
ergot medicines including:
- dihydroergotamine
- ergonovine
- ergonovine ergotamine
- methylergonovine
-
glecaprevir and pibrentasvir
-
indinavir
-
irinotecan
-
ivosidenib
-
lurasidone (when atazanavir capsules are used with ritonavir)
-
lomitapide
-
lovastatin
-
midazolam, when taken by mouth for sedation
-
nevirapine
-
phenobarbital
-
phenytoin
-
pimozide
-
quinidine (when atazanavir capsules are used with ritonavir)
-
rifampin
-
sildenafil, when used for the treatment of pulmonary arterial hypertension
-
simvastatin
-
St. John’s wort
-
triazolam
-
Before taking atazanavir capsules, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems
- have liver problems, including hepatitis B or C virus infection
- have kidney problems
- are receiving dialysis treatment
- have diabetes
- have hemophilia
- are pregnant or plan to become pregnant. *Atazanavir capsules must be taken with ritonavir during pregnancy. ***Hormonal forms of birth control, such as injections, vaginal rings or implants, contraceptive patch, and some birth control pills may not work during treatment with atazanavir capsules.**Talk to your healthcare provider about forms of birth control that may be used during treatment with atazanavir capsules. **Pregnancy Exposure Registry.There is a pregnancy exposure registry for people who take atazanavir capsules during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry. After your baby is born, tell your healthcare provider if your baby’s skin or the white part of their eyes turns yellow.
- are breastfeeding or plan to breastfeed.Do not breastfeed if you are taking atazanavir capsules.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby. Atazanavir can pass into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take**,** including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines interact with atazanavir capsules.Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with atazanavir capsules. ***Do not start taking a new medicine without telling your healthcare provider.**Your healthcare provider can tell you if it is safe to take atazanavir capsules with other medicines.
How should I take atazanavir capsules?
*Take atazanavir capsules exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking atazanavir capsules unless your healthcare provider tells you to.
- Stay under the care of your healthcare provider during treatment with atazanavir capsules.
- Atazanavir capsules must be used with other HIV-1 medicines.
- Take atazanavir capsules 1 time each day.
- Atazanavir comes as capsules.
- Take atazanavir capsules with food.
- Swallow the capsules whole. Do not open the capsules.
- Your child’s healthcare provider will prescribe the right dose of atazanavir capsules based on your child’s weight.
- If you miss a dose of atazanavir capsules, take it as soon as you remember. Then take the next dose at your regular time. Do not take 2 doses at the same time.
- If you take too much atazanavir, call your healthcare provider or go to the nearest hospital emergency room right away.
When your supply of atazanavir capsules starts to run low**,**get more from your healthcare provider or pharmacy. It is important not to run out of atazanavir capsules. The amount of HIV-1 in your blood may increase if the medicine is stopped for even a short time. The virus may become resistant to atazanavir capsules and harder to treat.
What are the possible side effects of atazanavir capsules?
Atazanavir capsules can cause serious side effects, including:
***A change in the way your heart beats (heart rhythm change).**Tell your healthcare provider right away if you get dizzy or lightheaded. These could be symptoms of a heart problem. *Skin rash. Skin rash is common with atazanavir capsules but can sometimes be severe. Severe rash may develop with other symptoms which could be serious. If you develop a severe rash or a rash with any of the following symptoms, stop taking atazanavir capsules and call your healthcare provider or go to the nearest hospital emergency room right away: * general feeling of discomfort or “flu-like” symptoms * fever * muscle or joint aches * red or inflamed eyes, like “pink eye” (conjunctivitis) * blisters * mouth sores * swelling of your face * painful, warm, or red lump under your skin ***Liver problems.**If you have liver problems, including hepatitis B or C infection, your liver problems may get worse when you take atazanavir capsules. Your healthcare provider will do blood tests to check your liver before you start atazanavir capsules and during treatment. Tell your healthcare provider right away if you get any of the following symptoms: * dark “tea-colored” urine * your skin or the white part of your eyes turns yellow * light colored stools * nausea * itching * stomach-area pain *Chronic kidney disease. Atazanavir capsules may affect how well your kidneys work. Your healthcare provider will do blood and urine tests to check your kidneys before you start atazanavir capsules and during treatment. Drink plenty of fluids during treatment with atazanavir capsules. *Kidney stones have happened in some people who take atazanavir capsules, and sometimes may lead to hospitalization. Tell your healthcare provider right away if you get symptoms of kidney stones which may include pain in your low back or low stomach area, blood in your urine, or pain when you urinate. *Gallbladder stoneshave happened in some people who take atazanavir capsules, and sometimes may lead to hospitalization. Tell your healthcare provider right away if you get symptoms of a gallbladder problem which may include: * pain in the right or middle upper stomach area * fever * nausea and vomiting * your skin or the white part of your eyes turns yellow *Yellowing of your skin or the white part of your eyes is common with atazanavir capsules but may be a symptom of a serious problem. These symptoms may be due to increases in bilirubin levels in your blood (bilirubin is made by the liver). Tell your healthcare provider right away if your skin or the white part of your eyes turns yellow. *New or worsening diabetes and high blood sugar (hyperglycemia) have happened in some people who take protease inhibitor medicines like atazanavir capsules. Some people have had to start taking medicine to treat diabetes or have changes to their dose of their diabetes medicine. Tell your healthcare provider if you notice an increase in thirst or if you start urinating more often while taking atazanavir capsules. ***Changes in your immune system (Immune Reconstitution Syndrome)**can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider if you start having new symptoms after starting atazanavir capsules. *Changes in body fatcan happen in people taking HIV-1 medicines. These changes may include increased amount of fat in the upper back and neck (“buffalo hump”), breast, and around the main part of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known. *Increased bleeding problems in people with hemophiliahave happened when taking protease inhibitors like atazanavir capsules.
The most common side effects of atazanavir capsules include:
- nausea
- headache
- stomach-area pain
- vomiting
- trouble sleeping
- numbness, tingling, or burning of hands or feet
- dizziness
- muscle pain
- diarrhea
- depression
- fever
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of atazanavir capsules. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store atazanavir capsules?
- Store atazanavir capsules at room temperature, between 20° to 25°C (68° to 77°F).
- Keep capsules in a tightly closed container.
Keep atazanavir capsules and all medicines out of the reach of children.
General information about the safe and effective use of atazanavir capsules
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use atazanavir capsules for a condition for which it was not prescribed. Do not give atazanavir capsules to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about atazanavir capsules that is written for health professionals.
For more information, call Northstar Rx LLC at 1-800-206-7821.
What are the ingredients in atazanavir capsules?
**Active ingredient:**atazanavir sulfate
**Inactive ingredients:**crospovidone, lactose monohydrate, and magnesium stearate. The capsule shells contain the following inactive ingredients: FD&C blue 2, gelatin, iron oxide black, iron oxide red, iron oxide yellow, and titanium dioxide. The capsules are printed with ink containing black iron oxide, potassium hydroxide, propylene glycol, shellac, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Manufactured for: Northstar Rx LLC
Memphis, TN 38141.
Manufactured by: Aurobindo Pharma Limited
Unit-VII (SEZ)
Mahabubnagar (Dt)-509302
India.
M.L.No.: 22/MN/AP/2009/F/R
Revised: 01/2024
Use In Specific Populations Section
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to atazanavir during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Atazanavir has been evaluated in a limited number of women during pregnancy. Available human and animal data suggest that atazanavir does not increase the risk of major birth defects overall compared to the background rate [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. No treatment-related malformations were observed in rats and rabbits, for which the atazanavir exposures were 0.7 to 1.2 times of those at the human clinical dose (300 mg/day atazanavir boosted with 100 mg/day ritonavir). When atazanavir was administered to rats during pregnancy and throughout lactation, reversible neonatal growth retardation was observed [see Data].
Clinical Considerations
Dose Adjustments during Pregnancy and the Postpartum Period
- Atazanavir must be administered with ritonavir in pregnant patients.
- For pregnant patients, no dosage adjustment is required for atazanavir with the following exceptions:
- For treatment-experienced pregnant women during the second or third trimester, when atazanavir is coadministered with either an H2-receptor antagonistortenofovir DF, atazanavir 400 mg with ritonavir 100 mg once daily is recommended. There are insufficient data to recommend a atazanavir dose for use with both an H2-receptor antagonist and tenofovir DF in treatment-experienced pregnant patients.
- No dosage adjustment is required for postpartum patients. However, patients should be closely monitored for adverse events because atazanavir exposures could be higher during the first 2 months after delivery [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
Maternal Adverse Reactions
Cases of lactic acidosis syndrome, sometimes fatal, and symptomatic hyperlactatemia have occurred in pregnant women using atazanavir in combination with nucleoside analogues, which are associated with an increased risk of lactic acidosis syndrome.
Hyperbilirubinemia occurs frequently in patients who take atazanavir [see Warnings and Precautions (5.8)], including those who are pregnant [see Data].
Advise pregnant women of the potential risks of lactic acidosis syndrome and hyperbilirubinemia.
Fetal/Neonatal Adverse Reactions
All infants, including neonates exposed to atazanavir in utero, should be monitored for the development of severe hyperbilirubinemia during the first few days of life [see Data].
Data
Human Data
In Study AI424-182, atazanavir with ritonavir (300/100 mg or 400/100 mg) coadministered with lamivudine/zidovudine (150 mg/ 300 mg, as fixed-dose product) was administered to 41 pregnant women with HIV-1 infection, during the second or third trimester. Among the 39 women who completed the study, 38 women achieved an HIV-1 RNA less than 50 copies/mL at time of delivery. Six of 20 (30%) women on atazanavir with ritonavir 300/100 mg and 13 of 21 (62%) women on atazanavir with ritonavir 400/100 mg experienced hyperbilirubinemia (total bilirubin greater than or equal to 2.6 times ULN). There were no cases of lactic acidosis observed in clinical trial AI424-182.
Atazanavir drug concentrations in fetal umbilical cord blood were approximately 12% to 19% of maternal concentrations. Among the 40 infants born to 40 pregnant women with HIV-1 infection, all had test results that were negative for HIV-1 DNA at the time of delivery and/or during the first 6 months postpartum. All 40 infants received antiretroviral prophylactic treatment containing zidovudine. No evidence of severe hyperbilirubinemia (total bilirubin levels greater than 20 mg/dL) or acute or chronic bilirubin encephalopathy was observed among neonates in this study. However, 10/36 (28%) infants (6 greater than or equal to 38 weeks gestation and 4 less than 38 weeks gestation) had bilirubin levels of 4 mg/dL or greater within the first day of life.
Lack of ethnic diversity was a study limitation. In the study population, 33/40 (83%) infants were Black/African American, who have a lower incidence of neonatal hyperbilirubinemia than Caucasians and Asians. In addition, women with Rh incompatibility were excluded, as well as women who had a previous infant who developed hemolytic disease and/or had neonatal pathologic jaundice (requiring phototherapy).
Additionally, of the 38 infants who had glucose samples collected in the first day of life, 3 had adequately collected serum glucose samples with values of less than 40 mg/dL that could not be attributed to maternal glucose intolerance, difficult delivery, or sepsis.
Based on prospective reports from the APR of approximately 1600 live births following exposure to atazanavir-containing regimens (including 1037 live births in infants exposed in the first trimester and 569 exposed in second/third trimesters), there was no difference between atazanavir, and overall birth defects compared with the background birth defect rate. In the U.S. general population, the estimated background risk of major birth defects in clinically recognized pregnancies is 2 to 4%.
Animal Data
In animal reproduction studies, there was no evidence of mortality or teratogenicity in offspring born to animals at systemic drug exposure levels (AUC) 0.7 (in rabbits) to 1.2 (in rats) times those observed at the human clinical dose (300 mg/day atazanavir boosted with 100 mg/day ritonavir). In pre- and postnatal development studies in the rat, atazanavir caused neonatal growth retardation during lactation that reversed after weaning. Maternal drug exposure at this dose was 1.3 times the human exposure at the recommended clinical exposure. Minimal maternal toxicity occurred at this exposure level.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that patients with HIV-1 infection, not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Atazanavir has been detected in human milk. No data are available regarding atazanavir effects on milk production. Atazanavir was present in the milk of lactating rats and was associated with neonatal growth retardation that reversed after weaning.
Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed.
8.4 Pediatric Use
Atazanavir capsule is indicated in combination with other antiretroviral agents for the treatment of pediatric patients with HIV-1 infection, 6 years of age and older weighing at least 15 kg. Atazanavir is not recommended for use in pediatric patients below the age of 3 months due to the risk of kernicterus [see Indications and Usage (1)]. All atazanavir contraindications, warnings, and precautions apply to pediatric patients [see Contraindications (4) and Warnings and Precautions (5)].
The safety, pharmacokinetic profile, and virologic response of atazanavir in pediatric patients at least 6 years of age and older weighing at least 15 kg were established in an open-label, multicenter clinical trial: PACTG 1020A [see Clinical Pharmacology (12.3) and Clinical Studies (14.3)]. The safety profile in pediatric patients was generally similar to that observed in adults [see Adverse Reactions (6.1)]. See Dosage and Administration (2.4) for dosing recommendations for the use of atazanavir capsules.
8.5 Geriatric Use
Clinical studies of atazanavir did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Based on a comparison of mean single-dose pharmacokinetic values for Cmax and AUC, a dose adjustment based upon age is not recommended. In general, appropriate caution should be exercised in the administration and monitoring of atazanavir in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Age/Gender
A study of the pharmacokinetics of atazanavir was performed in young (n=29; 18 to 40 years) and elderly (n=30; ≥65 years) healthy subjects. There were no clinically significant pharmacokinetic differences observed due to age or gender.
8.7 Impaired Renal Function
Atazanavir is not recommended for use in treatment-experienced patients with HIV-1 infection, who have end-stage renal disease managed with hemodialysis [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)].
8.8 Impaired Hepatic Function
Atazanavir is not recommended for use in patients with severe hepatic impairment. Atazanavir with ritonavir is not recommended in patients with any degree of hepatic impairment [see Dosage and Administration (2.8) and Clinical Pharmacology (12.3)].
- Pregnancy: Available human and animal data suggest that atazanavir does not increase the risk of major birth defects overall compared to the background rate. (8.1)
- Lactation: Breastfeeding is not recommended. (8.2)
- Hepatitis B or C co-infection: Monitor liver enzymes. (5.4, 6.1)
- Renal impairment: Atazanavir is not recommended for use in treatment-experienced patients with end-stage renal disease managed with hemodialysis. (2.7, 8.7)
- Hepatic impairment: Atazanavir is not recommended in patients with severe hepatic impairment. Atazanavir with ritonavir is not recommended in patients with any degree of hepatic impairment. (2.8, 8.8)
Overdosage Section
10 OVERDOSAGE
Human experience of acute overdose with atazanavir is limited. Single doses up to 1200 mg (three times the 400 mg maximum recommended dose) have been taken by healthy subjects without symptomatic untoward effects. A single self- administered overdose of 29.2 g of atazanavir in a patient with HIV-1 infection (73 times the 400 mg recommended dose) was associated with asymptomatic bifascicular block and PR interval prolongation. These events resolved spontaneously. At atazanavir doses resulting in high atazanavir exposures, jaundice due to indirect (unconjugated) hyperbilirubinemia (without associated liver function test changes) or PR interval prolongation may be observed [see Warnings and Precautions (5.1, 5.8) and Clinical Pharmacology (12.2)].
Treatment of overdosage with atazanavir should consist of general supportive measures, including monitoring of vital signs and ECG, and observations of the patient’s clinical status. If indicated, elimination of unabsorbed atazanavir should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid removal of unabsorbed drug. There is no specific antidote for overdose with atazanavir. Since atazanavir is extensively metabolized by the liver and is highly protein bound, dialysis is unlikely to be beneficial in significant removal of this medicine.
Description Section
11 DESCRIPTION
The active ingredient in atazanavir capsules is atazanavir sulfate, which is an HIV-1 protease inhibitor.
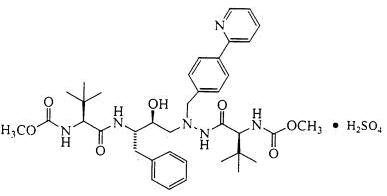
The chemical name for atazanavir sulfate is (3S,8S,9S,12S)-3,12-Bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester, sulfate (1:1). Its molecular formula is C38H52N6O7•H2SO4, which corresponds to a molecular weight of 802.9 (sulfuric acid salt). The free base molecular weight is 704.9. Atazanavir sulfate has the following structural formula:
Atazanavir sulfate is a white to pale-yellow crystalline powder. It is slightly soluble in water (4 to 5 mg/mL, free base equivalent) with the pH of a saturated solution in water being about 1.9 at 24 ± 3°C.
Atazanavir capsules are available for oral administration in strengths of 150 mg, 200 mg, or 300 mg of atazanavir, which are equivalent to 170.854 mg, 227.805 mg, or 341.708 mg of atazanavir sulfate, respectively. The capsules also contain the following inactive ingredients: crospovidone, lactose monohydrate, and magnesium stearate. The capsule shells contain the following inactive ingredients: FD&C blue 2, gelatin, iron oxide black, iron oxide red, iron oxide yellow, and titanium dioxide. The capsules are printed with ink containing black iron oxide, potassium hydroxide, propylene glycol, shellac, and titanium dioxide.
Clinical Pharmacology Section
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atazanavir is an HIV-1 antiretroviral drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
Concentration- and dose-dependent prolongation of the PR interval in the electrocardiogram has been observed in healthy subjects receiving atazanavir. In placebo-controlled Study AI424-076, the mean (±SD) maximum change in PR interval from the predose value was 24 (±15) msec following oral dosing with 400 mg of atazanavir (n=65) compared to 13 (±11) msec following dosing with placebo (n=67). The PR interval prolongations in this study were asymptomatic. There is limited information on the potential for a pharmacodynamic interaction in humans between atazanavir and other drugs that prolong the PR interval of the electrocardiogram [see Warnings and Precautions (5.1)].
Electrocardiographic effects of atazanavir were determined in a clinical pharmacology study of 72 healthy subjects. Oral doses of 400 mg (maximum recommended dosage) and 800 mg (twice the maximum recommended dosage) were compared with placebo; there was no concentration- dependent effect of atazanavir on the QTc interval (using Fridericia’s correction). In 1793 subjects with HIV-1 infection, receiving antiretroviral regimens, QTc prolongation was comparable in the atazanavir and comparator regimens. No atazanavir-treated healthy subject or subject with HIV-1 infection in clinical trials had a QTc interval >500 msec [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
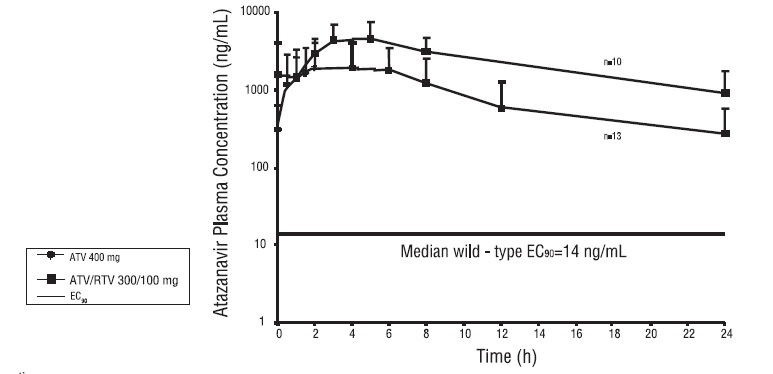
The pharmacokinetics of atazanavir were evaluated in adult subjects who either were healthy, or with HIV infection, after administration of atazanavir 400 mg once daily and after administration of atazanavir 300 mg with ritonavir 100 mg once daily (see Table 17).
Table 17: Steady-State Pharmacokinetics of Atazanavir in Healthy Subjects or Subjects with HIV-1 Infection in the Fed State|
400 mg once daily |
300 mg with ritonavir | |||
|
Parameter |
Healthy |
Subjects with HIV-1 Infection** (n=13)** |
Healthy |
Subjects with HIV-1 Infection** (n=10)** |
|
Cmax (ng/mL) | ||||
|
Geometric mean (CV%) |
5199 (26) |
2298 (71) |
6129 (31) |
4422 (58) |
|
Mean (SD) |
5358 (1371) |
3152 (2231) |
6450 (2031) |
5233 (3033) |
|
Tmax (h) | ||||
|
Median |
2.5 |
2.0 |
2.7 |
3.0 |
|
AUC (ng•h/mL) | ||||
|
Geometric mean (CV%) |
28132 (28) |
14874 (91) |
57039 (37) |
46073 (66) |
|
Mean (SD) |
29303 (8263) |
22262 (20159) |
61435 (22911) |
53761 (35294) |
|
T-half (h) | ||||
|
Mean (SD) |
7.9 (2.9) |
6.5 (2.6) |
18.1 (6.2)a |
8.6 (2.3) |
|
Cmin (ng/mL) | ||||
|
Geometric mean (CV%) |
159 (88) |
120 (109) |
1227 (53) |
636 (97) |
|
Mean (SD) |
218 (191) |
273 (298)b |
1441 (757) |
862 (838) |
|
a n=26. |
Figure 1 displays the mean plasma concentrations of atazanavir at steady state after atazanavir 400 mg once daily (as two 200 mg capsules) with a light meal and after atazanavir 300 mg (as two 150 mg capsules) with ritonavir 100 mg once daily with a light meal in adult subjects with HIV-1 infection.
** Figure 1: Mean (SD) Steady-State Plasma Concentrations of Atazanavir 400 mg
(n=13) and 300 mg with Ritonavir (n=10) for HIV-Infected Adult Subjects with
HIV-1 Infection**
****
Absorption
Atazanavir is rapidly absorbed with a Tmax of approximately 2.5 hours. Atazanavir demonstrates nonlinear pharmacokinetics with greater than dose- proportional increases in AUC and Cmax values over the dose range of 200 to 800 mg once daily. Steady state is achieved between Days 4 and 8, with an accumulation of approximately 2.3-fold.
Food Effect
Administration of atazanavir with food enhances bioavailability and reduces pharmacokinetic variability. Administration of a single 400 mg dose of atazanavir with a light meal (357 kcal, 8.2 g fat, 10.6 g protein) resulted in a 70% increase in AUC and 57% increase in Cmax relative to the fasting state. Administration of a single 400 mg dose of atazanavir with a high-fat meal (721 kcal, 37.3 g fat, 29.4 g protein) resulted in a mean increase in AUC of 35% with no change in Cmax relative to the fasting state. Administration of atazanavir with either a light meal or high-fat meal decreased the coefficient of variation of AUC and Cmax by approximately one-half compared to the fasting state.
Coadministration of a single 300 mg dose of atazanavir and a 100 mg dose of ritonavir with a light meal (336 kcal, 5.1 g fat, 9.3 g protein) resulted in a 33% increase in the AUC and a 40% increase in both the Cmax and the 24-hour concentration of atazanavir relative to the fasting state. Coadministration with a high-fat meal (951 kcal, 54.7 g fat, 35.9 g protein) did not affect the AUC of atazanavir relative to fasting conditions and the Cmax was within 11% of fasting values. The 24-hour concentration following a high-fat meal was increased by approximately 33% due to delayed absorption; the median Tmax increased from 2.0 to 5.0 hours. Coadministration of atazanavir with ritonavir with either a light or a high-fat meal decreased the coefficient of variation of AUC and Cmax by approximately 25% compared to the fasting state.
Distribution
Atazanavir is 86% bound to human serum proteins and protein binding is independent of concentration. Atazanavir binds to both alpha-1-acid glycoprotein (AAG) and albumin to a similar extent (89% and 86%, respectively). In a multiple-dose study in subjects with HIV-1 infection dosed with atazanavir 400 mg once daily with a light meal for 12 weeks, atazanavir was detected in the cerebrospinal fluid and semen. The cerebrospinal fluid/plasma ratio for atazanavir (n=4) ranged between 0.0021 and 0.0226 and seminal fluid/plasma ratio (n=5) ranged between 0.11 and 4.42.
Metabolism
Atazanavir is extensively metabolized in humans. The major biotransformation pathways of atazanavir in humans consisted of monooxygenation and dioxygenation. Other minor biotransformation pathways for atazanavir or its metabolites consisted of glucuronidation, N-dealkylation, hydrolysis, and oxygenation with dehydrogenation. Two minor metabolites of atazanavir in plasma have been characterized. Neither metabolite demonstrated in vitro antiviral activity. In vitro studies using human liver microsomes suggested that atazanavir is metabolized by CYP3A.
Elimination
Following a single 400 mg dose of 14C-atazanavir, 79% and 13% of the total radioactivity was recovered in the feces and urine, respectively. Unchanged drug accounted for approximately 20% and 7% of the administered dose in the feces and urine, respectively. The mean elimination half-life of atazanavir in healthy subjects (n=214) and adult subjects with HIV-1 infection (n=13) was approximately 7 hours at steady state following a dose of 400 mg daily with a light meal.
Specific Populations
Renal Impairment
In healthy subjects, the renal elimination of unchanged atazanavir was approximately 7% of the administered dose. Atazanavir has been studied in adult subjects with severe renal impairment (n=20), including those on hemodialysis, at multiple doses of 400 mg once daily. The mean atazanavir Cmax was 9% lower, AUC was 19% higher, and Cmin was 96% higher in subjects with severe renal impairment not undergoing hemodialysis (n=10), than in age-, weight-, and gender-matched subjects with normal renal function. In a 4-hour dialysis session, 2.1% of the administered dose was removed. When atazanavir was administered either prior to, or following hemodialysis (n=10), the geometric means for Cmax, AUC, and Cmin were approximately 25% to 43% lower compared to subjects with normal renal function. The mechanism of this decrease is unknown. Atazanavir is not recommended for use in treatment- experienced patients with HIV-1 who have end-stage renal disease managed with hemodialysis [see Dosage and Administration (2.7)].
Hepatic Impairment
Atazanavir has been studied in adult subjects with moderate-to-severe hepatic impairment (14 Child-Pugh B and 2 Child-Pugh C subjects) after a single 400 mg dose. The mean AUC(0-∞) was 42% greater in subjects with impaired hepatic function than in healthy subjects. The mean half-life of atazanavir in hepatically impaired subjects was 12.1 hours compared to 6.4 hours in healthy subjects. A dose reduction to 300 mg is recommended for patients with moderate hepatic impairment (Child-Pugh Class B) who have not experienced prior virologic failure as increased concentrations of atazanavir are expected. Atazanavir is not recommended for use in patients with severe hepatic impairment. The pharmacokinetics of atazanavir in combination with ritonavir has not been studied in subjects with hepatic impairment; thus, coadministration of atazanavir with ritonavir is not recommended for use in patients with any degree of hepatic impairment [see Dosage and Administration (2.8)].
Pediatrics
The pharmacokinetic parameters for atazanavir at steady state in pediatric subjects taking the capsule formulation were predicted by a population pharmacokinetic model and are summarized in Table 19 by weight ranges that correspond to the recommended doses [see Dosage and Administration (2.4)].
Table 19: Predicted Steady-State Pharmacokinetics of Atazanavir (capsule formulation) with Ritonavir in Pediatric Subjects with HIV-1 Infection|
Body Weight |
atazanavir with ritonavir Dose (mg) |
Cmax ng/mL Geometric Mean |
AUC ng•h/mL Geometric Mean |
Cmin ng/mL Geometric Mean (CV%) |
|
15 to <35 |
200/100 |
3303 (86%) |
37235 (84%) |
538 (99%) |
|
≥35 |
300/100 |
2980 (82%) |
37643 (83%) |
653 (89%) |
Pregnancy
The pharmacokinetic data from pregnant women with HIV-1 infection receiving atazanavir capsules with ritonavir are presented in Table 20.
Table 20: Steady-State Pharmacokinetics of Atazanavir with Ritonavir in Pregnant Women with HIV-1 Infection in the Fed State|
Pharmacokinetic Parameter |
Atazanavir 300 mg with ritonavir 100 mg | ||
|
2nd Trimester |
3rd Trimester |
Postpartum****b | |
|
Cmax ng/mL |
3078.85 |
3291.46 |
5721.21 |
|
Geometric mean (CV%) |
(50) |
(48) |
(31) |
|
AUC ng•h/mL |
27657.1 |
34251.5 |
61990.4 |
|
Geometric mean (CV%) |
(43) |
(43) |
(32) |
|
Cmin ng/mLc |
538.70 |
668.48 |
1462.59 |
|
Geometric mean (CV%) |
(46) |
(50) |
(45) |
|
a Available data during the 2nd trimester are limited. |
Drug Interaction Data
Atazanavir is a metabolism-dependent CYP3A inhibitor, with a Kinact value of 0.05 to 0.06 min-1 and Ki value of 0.84 to 1.0 µM. Atazanavir is also a direct inhibitor for UGT1A1 (Ki=1.9 µM) and CYP2C8 (Ki=2.1 µM).
Atazanavir has been shown in vivo not to induce its own metabolism nor to increase the biotransformation of some drugs metabolized by CYP3A. In a multiple-dose study, atazanavir decreased the urinary ratio of endogenous 6β-OH cortisol to cortisol versus baseline, indicating that CYP3A production was not induced.
Clinically significant interactions are not expected between atazanavir and substrates of CYP2C19, CYP2C9, CYP2D6, CYP2B6, CYP2A6, CYP1A2, or CYP2E1. Clinically significant interactions are not expected between atazanavir when administered with ritonavir and substrates of CYP2C8. See the complete prescribing information for ritonavir for information on other potential drug interactions with ritonavir.
Based on known metabolic profiles, clinically significant drug interactions are not expected between atazanavir and dapsone, trimethoprim/sulfamethoxazole, azithromycin, or erythromycin. Atazanavir does not interact with substrates of CYP2D6 (e.g., nortriptyline, desipramine, metoprolol).
Drug interaction studies were performed with atazanavir and other drugs likely to be coadministered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration of atazanavir on the AUC, Cmax, and Cmin are summarized in Tables 21 and 22. Neither didanosine EC nor diltiazem had a significant effect on atazanavir exposures (see Table 22 for effect of atazanavir on didanosine EC or diltiazem exposures). Atazanavir did not have a significant effect on the exposures of didanosine (when administered as the buffered tablet), stavudine, or fluconazole. For information regarding clinical recommendations, see Drug Interactions (7).
Table 21: Drug Interactions: Pharmacokinetic Parameters for Atazanavir in the Presence of Coadministered Drugsa|
Coadministered Drug |
Coadministered Drug Dose/Schedule |
Atazanavir****Dose/Schedule |
Ratio (90% Confidence Interval) of Atazanavir Pharmacokinetic Parameters
with/without Coadministered Drug; | ||
|
C****max |
AUC |
C****min | |||
|
atenolol |
50 mg QD, d 7 to 11 |
400 mg QD, d 1 to 11 |
1.00 |
0.93 |
0.74 |
|
clarithromycin |
500 mg BID, d 7 to 10 |
400 mg QD, d 1 to 10 |
1.06 |
1.28 |
1.91 |
|
didanosine (ddI) |
ddI: 200 mg x 1 dose, |
400 mg x 1 dose |
0.11 |
0.13 |
0.16 |
|
ddI: 200 mg x 1 dose, |
400 mg x 1 dose |
1.12 |
1.03 |
1.03 | |
|
efavirenz |
600 mg QD, d 7 to 20 (n=27) |
400 mg QD, d 1 to 20 |
0.41 |
0.26 |
0.07 |
|
600 mg QD, d 7 to 20 |
400 mg QD, d 1 to 6 (n=23) |
1.14 |
1.39 |
1.48 | |
|
600 mg QD, |
300 mg QD with ritonavir |
1.17 |
1.00 |
0.58 | |
|
famotidine |
40 mg BID, |
400 mg QD, d 1 to 6 (n=45), |
0.53 |
0.59 |
0.58 |
|
40 mg BID, d 7 to 12 |
400 mg QD (pm), d 1 to 6 |
1.08 |
0.95 |
0.79 | |
|
40 mg BID, d 11 to 20 |
300 mg QD with ritonavir |
0.86 |
0.82 |
0.72 | |
|
20 mg BID, d 11 to 17 |
300 mg QD with ritonavir |
0.91 |
0.90 |
0.81 | |
|
40 mg QD (pm), |
300 mg QD with ritonavir |
0.89 |
0.88 |
0.77 | |
|
40 mg BID, |
300 mg QD with ritonavir |
0.74 |
0.79 |
0.72 | |
|
40 mg BID, |
300 mg QD with ritonavir |
1.02 |
1.03 |
0.86 | |
|
grazoprevir/elbasvir |
grazoprevir 200 mg QD d 1 to 35 (n = 11) |
300 mg QD with ritonavir |
1.12 |
1.43 |
1.23 |
|
elbasvir 50 mg QD |
300 mg QD with ritonavir |
1.02 |
1.07 |
1.15 | |
|
ketoconazole |
200 mg QD, |
400 mg QD, d 1 to 13 |
0.99 |
1.10 |
1.03 |
|
nevirapinef,g |
200 mg BID, d 1 to 23 |
300 mg QD with ritonavir |
0.72 1.02 |
0.58 0.81 |
0.28 0.41 |
|
omeprazole |
40 mg QD, d 7 to 12 40 mg QD, d 11 to 20 20 mg QD, d 17 to 23 20 mg QD, d 17 to 23 |
400 mg QD, d 1 to 6 (n=48), 300 mg QD with ritonavir 300 mg QD with ritonavir 300 mg QD with ritonavir |
0.04 0.28 0.61 0.69 |
0.06 0.24 0.58 0.70 |
0.05 0.22 0.54 0.69 |
|
pitavastatin |
4 mg QD |
300 mg QD |
1.13 |
1.06 |
NA |
|
rifabutin |
150 mg QD, |
400 mg QD, d 1 to 28 |
1.34 |
1.15 |
1.13 |
|
rifampin |
600 mg QD, |
300 mg QD with ritonavir |
0.47 |
0.28 |
0.02 |
|
ritonavirn |
100 mg QD, |
300 mg QD, d 1 to 20 |
1.86 |
3.38 |
11.89 |
|
tenofovir DFo |
300 mg QD, d 9 to 16 |
400 mg QD, d 2 to 16 |
0.79 |
0.75 |
0.60 |
|
300 mg QD, |
300 mg with ritonavir 100 mg |
0.72p |
0.75p |
0.77p | |
|
voriconazole |
200 mg BID, |
300 mg with ritonavir 100 mg |
0.87 |
0.88 |
0.80 |
|
voriconazole |
50 mg BID, |
300 mg with ritonavir 100 mg |
0.81 |
0.80 |
0.69 |
|
a Data provided are under fed conditions unless otherwise noted. |
|
Coadministered Drug |
Coadministered Drug Dose/Schedule |
Atazanavir****Dose/Schedule |
Ratio (90% Confidence Interval) of Coadministered Drug Pharmacokinetic
Parameters with/withoutAtazanavir; | ||
|
C****max |
AUC |
C****min | |||
|
acetaminophen |
1 g BID, d 1 to 20 |
300 mg QD with ritonavir |
0.87 |
0.97 |
1.26 |
|
atenolol |
50 mg QD, d 7 to 11 |
400 mg QD, d 1 to 11 |
1.34 |
1.25 |
1.02 |
|
clarithromycin |
500 mg BID, |
400 mg QD, |
1.50 |
1.94 |
2.60 |
|
ddI |
400 mg d 1 |
400 mg QD, d 2 to 8 |
0.64 |
0.66 |
1.13 |
|
400 mg d 1 |
300 mg QD with ritonavir |
0.62 |
0.66 |
1.25 | |
|
diltiazem |
180 mg QD, |
400 mg QD, d 1 to 11 |
1.98 |
2.25 |
2.42 |
|
ethinyl estradiol |
Ortho-Novum® |
400 mg QD, |
ethinyl estradiol: 1.15 |
ethinyl estradiol: 1.48 |
ethinyl estradiol: 1.91 |
|
ethinyl estradiol & norgestimated |
Ortho Tri-Cyclen® |
300 mg QD with ritonavir |
ethinyl estradiol: 0.84 |
ethinyl estradiol: 0.81 |
ethinyl estradiol: 0.63 |
|
glecaprevir/ pibrentasvir |
300 mg glecaprevir (n=12) |
300 mg QD with ritonavir 100 mg QD |
≥4.06g |
≥6.53g |
≥14.3g |
|
120 mg pibrentasvir (n=12) |
300 mg QD with ritonavir 100 mg QD |
≥1.29g |
≥1.64g |
≥2.29g | |
|
grazoprevir/ elbasvir |
grazoprevir 200 mg QD d 1 to 35 (n=12) |
300 mg QD with ritonavir 100 mg QD |
6.24 |
10.58 |
11.64 |
|
elbasvir 50 mg QD |
300 mg QD with ritonavir 100 mg QD |
4.15 |
4.76 |
6.45 | |
|
methadone |
Stable maintenance |
400 mg QD, |
(R)-methadoneh |
(R)-methadoneh |
(R)-methadoneh |
|
nevirapinei,j |
200 mg BID, |
300 mg QD with ritonavir 100 mg |
1.17 1.21 |
1.25 1.26 |
1.32 1.35 |
|
omeprazolek |
40 mg single dose, |
400 mg QD, d 1 to 12 |
1.24 |
1.45 |
NA |
|
rifabutin |
300 mg QD, |
600 mg QD,l |
1.18 |
2.10 |
3.43 |
|
150 mg twice |
300 mg QD with ritonavir |
2.49m (2.03, 3.06) 25-O-desacetyl-rifabutin: 7.77 (6.13, 9.83) |
1.48m (1.19, 1.84) 25-O-desacetyl-rifabutin: 10.90 |
1.40m (1.05, 1.87) 25-O-desacetyl-rifabutin: 11.45 (8.15, 16.10) | |
|
pitavastatin |
4 mg QD |
300 mg QD |
1.60 |
1.31 |
NA |
|
rosiglitazonen |
4 mg single dose, |
400 mg QD, |
1.08 |
1.35 |
NA NA |
|
rosuvastatin |
10 mg single dose |
300 mg QD with ritonavir 100 mg |
↑ 7-foldo |
↑ 3-foldo |
NA |
|
saquinavirp (soft |
1200 mg QD, |
400 mg QD, d 7 to 13 |
4.39 |
5.49 |
6.86 |
|
sofosbuvir/ velpatasvir/ voxilaprevir |
400 mg sofosbuvir single dose |
300 mg with 100 mg ritonavir single dose (n=15) |
1.29 |
1.40 |
NA |
|
100 mg velpatasvir single dose |
300 mg with 100 mg ritonavir single dose (n=15) |
1.29 |
1.93 |
NA | |
|
100 mg voxilaprevir single dose |
300 mg with 100 mg ritonavir single dose (n=15) |
4.42 |
4.31 |
NA | |
|
tenofovir DFq |
300 mg QD, |
400 mg QD, d 2 to 16 |
1.14 |
1.24 |
1.22 |
|
300 mg QD, d 1 to 7 |
300 mg QD with ritonavir |
1.34 |
1.37 |
1.29 | |
|
voriconazole |
200 mg BID, |
300 mg with ritonavir |
0.90 |
0.67 |
0.61 |
|
voriconazole |
50 mg BID, |
300 mg with ritonavir |
4.38 |
5.61 |
7.65 |
|
lamivudine and zidovudine |
150 mg lamivudine and 300 mg zidovudine |
400 mg QD, d 7 to 12 |
lamivudine: 1.04 |
lamivudine: 1.03 |
lamivudine: 1.12 |
|
a Data provided are under fed conditions unless otherwise noted. |
12.4 Microbiology
Mechanism of Action
Atazanavir (ATV) is an azapeptide HIV-1 protease inhibitor (PI). The compound selectively inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.
Antiviral Activity in Cell Culture
Atazanavir exhibits anti-HIV-1 activity with a mean 50% effective concentration (EC50) in the absence of human serum of 2 to 5 nM against a variety of laboratory and clinical HIV-1 isolates grown in peripheral blood mononuclear cells, macrophages, CEM-SS cells, and MT-2 cells. Atazanavir has activity against HIV-1 Group M subtype viruses A, B, C, D, AE, AG, F, G, and J isolates in cell culture. Atazanavir has variable activity against HIV-2 isolates (1.9 to 32 nM), with EC50 values above the EC50 values of failure isolates. Two-drug combination antiviral activity studies with atazanavir showed no antagonism in cell culture with PIs (amprenavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir), NNRTIs (delavirdine, efavirenz, and nevirapine), NRTIs (abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir DF, and zidovudine), the HIV-1 fusion inhibitor enfuvirtide, and two compounds used in the treatment of viral hepatitis, adefovir and ribavirin, without enhanced cytotoxicity.
Resistance
In Cell Culture: HIV-1 isolates with a decreased susceptibility to atazanavir have been selected in cell culture and obtained from patients treated with atazanavir or atazanavir with ritonavir. HIV-1 isolates with 93- to 183-fold reduced susceptibility to atazanavir from three different viral strains were selected in cell culture by 5 months. The substitutions in these HIV-1 viruses that contributed to atazanavir resistance include I50L, N88S, I84V, A71V, and M46I. Changes were also observed at the protease cleavage sites following drug selection. Recombinant viruses containing the I50L substitution without other major PI substitutions were growth impaired and displayed increased susceptibility in cell culture to other PIs (amprenavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir). The I50L and I50V substitutions yielded selective resistance to atazanavir and amprenavir, respectively, and did not appear to be cross-resistant.
Clinical Studies of Treatment-Naive Subjects: Comparison of Ritonavir-Boosted Atazanavir vs. Unboosted Atazanavir: Study AI424-089 compared atazanavir 300 mg once daily with ritonavir 100 mg vs. Atazanavir 400 mg once daily when administered with lamivudine and extended- release stavudine in treatment- naive subjects with HIV-1 infection. A summary of the number of virologic failures and virologic failure isolates with atazanavir resistance in each arm is shown in Table 23.
Table 23: Summary of Virologic Failuresa at Week 96 in Study AI424-089: Comparison of Ritonavir Boosted Atazanavir vs. Unboosted Atazanavir: Randomized Subjects|
atazanavir |
atazanavir | |
|
Virologic Failure (≥50 copies/mL) at Week 96 |
15 (16%) |
34 (32%) |
|
Virologic Failure with Genotypes and Phenotypes Data |
5 |
17 |
|
Virologic Failure Isolates with atazanavir-resistance at Week 96 |
0/5 (0%)b |
4/17 (24%)b |
|
Virologic Failure Isolates with I50L Emergence at Week 96c |
0/5 (0%)b |
2/17 (12%)b |
|
Virologic Failure Isolates with Lamivudine Resistance at Week 96 |
2/5 (40%)b |
11/17 (65%)b |
|
a Virologic failure includes subjects who were never suppressed through Week
96 and on study at Week 96, had virologic rebound or discontinued due to
insufficient viral load response. |
Clinical Studies of Treatment-Naive Subjects Receiving Atazanavir 300 mg with Ritonavir 100 mg: In Phase 3 Study AI424-138, an as-treated genotypic and phenotypic analysis was conducted on samples from subjects who experienced virologic failure (HIV-1 RNA ≥400 copies/mL) or discontinued before achieving suppression on atazanavir with ritonavir (n=39; 9%) and lopinavir/ritonavir (n=39; 9%) through 96 weeks of treatment. In the atazanavir with ritonavir arm, one of the virologic failure isolates had a 56-fold decrease in atazanavir susceptibility emerge on therapy with the development of PI resistance-associated substitutions L10F, V32I, K43T, M46I, A71I, G73S, I85I/V, and L90M. The NRTI resistance-associated substitution M184V also emerged on treatment in this isolate conferring emtricitabine resistance. Two atazanavir with ritonavir-virologic failure isolates had baseline phenotypic atazanavir resistance and IAS-defined major PI resistance-associated substitutions at baseline. The I50L substitution emerged on study in one of these failure isolates and was associated with a 17-fold decrease in atazanavir susceptibility from baseline and the other failure isolate with baseline atazanavir resistance and PI substitutions (M46M/I and I84I/V) had additional IAS-defined major PI substitutions (V32I, M46I, and I84V) emerge on atazanavir treatment associated with a 3-fold decrease in atazanavir susceptibility from baseline. Five of the treatment failure isolates in the atazanavir with ritonavir arm developed phenotypic emtricitabine resistance with the emergence of either the M184I (n=1) or the M184V (n=4) substitution on therapy and none developed phenotypic tenofovir disoproxil resistance. In the lopinavir/ritonavir arm, one of the virologic failure subject isolates had a 69-fold decrease in lopinavir susceptibility emerge on therapy with the development of PI substitutions L10V, V11I, I54V, G73S, and V82A in addition to baseline PI substitutions L10L/I, V32I, I54I/V, A71I, G73G/S, V82V/A, L89V, and L90M. Six lopinavir/ritonavir virologic failure isolates developed the M184V substitution and phenotypic emtricitabine resistance and two developed phenotypic tenofovir disoproxil resistance.
Clinical Studies of Treatment-Naive Subjects Receiving Atazanavir 400 mg without Ritonavir: atazanavir-resistant clinical isolates from treatment-naive subjects who experienced virologic failure on atazanavir 400 mg treatment without ritonavir often developed an I50L substitution (after an average of 50 weeks of atazanavir therapy), often in combination with an A71V substitution, but also developed one or more other PI substitutions (e.g., V32I, L33F, G73S, V82A, I85V, or N88S) with or without the I50L substitution. In treatment-naive subjects, viral isolates that developed the I50L substitution, without other major PI substitutions, showed phenotypic resistance to atazanavir but retained in cell culture susceptibility to other PIs (amprenavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir); however, there are no clinical data available to demonstrate the effect of the I50L substitution on the efficacy of subsequently administered PIs.
Clinical Studies of Treatment-Experienced Subjects: In studies of treatment- experienced subjects treated with atazanavir or atazanavir with ritonavir, most atazanavir-resistant isolates from subjects who experienced virologic failure developed substitutions that were associated with resistance to multiple PIs and displayed decreased susceptibility to multiple PIs. The most common protease substitutions to develop in the viral isolates of subjects who failed treatment with atazanavir 300 mg once daily and ritonavir 100 mg once daily (together with tenofovir DF and an NRTI) included V32I, L33F/V/I, E35D/G, M46I/L, I50L, F53L/V, I54V, A71V/T/I, G73S/T/C, V82A/T/L, I85V, and L89V/Q/M/T. Other substitutions that developed on atazanavir with ritonavir treatment including E34K/A/Q, G48V, I84V, N88S/D/T, and L90M occurred in less than 10% of subject isolates. Generally, if multiple PI resistance substitutions were present in the HIV-1 virus of the subject at baseline, atazanavir resistance developed through substitutions associated with resistance to other PIs and could include the development of the I50L substitution. The I50L substitution has been detected in treatment-experienced subjects experiencing virologic failure after long-term treatment. Protease cleavage site changes also emerged on atazanavir treatment, but their presence did not correlate with the level of atazanavir resistance.
Cross-Resistance
Cross-resistance among PIs has been observed. Baseline phenotypic and genotypic analyses of clinical isolates from atazanavir clinical trials of PI- experienced subjects showed that isolates cross-resistant to multiple PIs were cross-resistant to atazanavir. Greater than 90% of the isolates with substitutions that included I84V or G48V were resistant to atazanavir. Greater than 60% of isolates containing L90M, G73S/T/C, A71V/T, I54V, M46I/L, or a change at V82 were resistant to atazanavir, and 38% of isolates containing a D30N substitution in addition to other changes were resistant to atazanavir. Isolates resistant to atazanavir were also cross-resistant to other PIs with
90% of the isolates resistant to indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir, and 80% resistant to amprenavir. In treatment-experienced subjects, PI-resistant viral isolates that developed the I50L substitution in addition to other PI resistance-associated substitution were also cross- resistant to other PIs.
Baseline Genotype/Phenotype and Virologic Outcome Analyses
Genotypic and/or phenotypic analysis of baseline virus may aid in determining atazanavir susceptibility before initiation of atazanavir with ritonavir therapy. An association between virologic response at 48 weeks and the number and type of primary PI resistance-associated substitutions detected in baseline HIV-1 isolates from antiretroviral-experienced subjects receiving atazanavir with ritonavir once daily or lopinavir/ritonavir (fixed-dose product) twice daily in Study AI424-045 is shown in Table 24.
Overall, both the number and type of baseline PI substitutions affected response rates in treatment-experienced subjects. In the atazanavir with ritonavir group, subjects had lower response rates when 3 or more baseline PI substitutions, including a substitution at position 36, 71, 77, 82, or 90, were present compared to subjects with 1 to 2 PI substitutions, including one of these substitutions.
Table 24: HIV-1 RNA Response by Number and Type of Baseline PI Substitution, Antiretroviral-Experienced Subjects in Study AI424-045, As- Treated Analysis|
Number and Type of Baseline PI Substitutions****a |
Virologic Response = HIV RNA <400 copies/mL****b | |
|
atazanavir with ritonavir (n=110) |
lopinavir/ritonavir**c** | |
|
3 or more primary PI substitutions including** d****:** | ||
|
D30N |
75% (6/8) |
50% (3/6) |
|
M36I/V |
19% (3/16) |
33% (6/18) |
|
M46I/L/T |
24% (4/17) |
23% (5/22) |
|
I54V/L/T/M/A |
31% (5/16) |
31% (5/16) |
|
A71V/T/I/G |
34% (10/29) |
39% (12/31) |
|
G73S/A/C/T |
14% (1/7) |
38% (3/8) |
|
V77I |
47% (7/15) |
44% (7/16) |
|
V82A/F/T/S/I |
29% (6/21) |
27% (7/26) |
|
I84V/A |
11% (1/9) |
33% (2/6) |
|
N88D |
63% (5/8) |
67% (4/6) |
|
L90M |
10% (2/21) |
44% (11/25) |
|
Number of baseline primary PI substitutions****a | ||
|
All patients, as-treated |
58% (64/110) |
59% (67/113) |
|
0 to 2 PI substitutions |
75% (50/67) |
75% (50/67) |
|
3 to 4 PI substitutions |
41% (14/34) |
43% (12/28) |
|
5 or more PI substitutions |
0% (0/9) |
28% (5/18) |
|
a Primary substitutions include any change at D30, V32, M36, M46, I47, G48,
I50, I54, A71, G73, V77, V82, I84, N88, and L90. |
The response rates of antiretroviral-experienced subjects in Study AI424-045 were analyzed by baseline phenotype (shift in susceptibility in cell culture relative to reference, Table 25). The analyses are based on a select population with 62% of subjects receiving an NNRTI-based regimen before study entry compared to 35% receiving a PI-based regimen. Additional data are needed to determine clinically relevant break points for atazanavir.
Table 25: Baseline Phenotype by Outcome, Antiretroviral-Experienced Subjects in Study AI424-045, As-Treated Analysis|
Baseline Phenotype****a |
Virologic Response = HIV-1 RNA <400 copies/mL****b | |
|
atazanavir with ritonavir (n=111) |
lopinavir/ritonavir**c** | |
|
0 to 2 |
71% (55/78) |
70% (56/80) |
|
53% (8/15) |
44% (4/9) |
|
13% (1/8) |
33% (3/9) |
|
10% (1/10) |
23% (3/13) |
|
a Fold change susceptibility in cell culture relative to the wild-type
reference. |
Nonclinical Toxicology Section
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies in mice and rats were carried out with atazanavir for two years. In the mouse study, drug-related increases in hepatocellular adenomas were found in females at 360 mg/kg/day. The systemic drug exposure (AUC) at the NOAEL (no observable adverse effect level) in females, (120 mg/kg/day) was 2.8 times and in males (80 mg/kg/day) was 2.9 times higher than those in humans at the clinical dose (300 mg/day atazanavir boosted with 100 mg/day ritonavir, non-pregnant patients). In the rat study, no drug-related increases in tumor incidence were observed at doses up to 1200 mg/kg/day, for which AUCs were 1.1 (males) or 3.9 (females) times those measured in humans at the clinical dose.
Mutagenesis
Atazanavir tested positive in an in vitro clastogenicity test using primary human lymphocytes, in the absence and presence of metabolic activation. Atazanavir tested negative in the in vitro Ames reverse-mutation assay, in vivo micronucleus and DNA repair tests in rats, and in vivo DNA damage test in rat duodenum (comet assay).
Impairment of Fertility
At the systemic drug exposure levels (AUC) 0.9 (in male rats) or 2.3 (in female rats) times that of the human clinical dose, (300 mg/day atazanavir boosted with 100 mg/day ritonavir) significant effects on mating, fertility, or early embryonic development were not observed.
Clinical Studies Section
14 CLINICAL STUDIES
14.1 Adult Subjects without Prior Antiretroviral Therapy
Study AI424-138: a 96-week study comparing the antiviral efficacy and safety of either atazanavir or lopinavir/ritonavir, each in combination with fixed- dose tenofovir DF-emtricitabine in treatment-naive subjects with HIV-1 infection. Study AI424-138 (NCT00272779) was a 96-week, open-label, randomized, multicenter study, comparing atazanavir (300 mg once daily) with ritonavir (100 mg once daily) to lopinavir/ritonavir (400/100 mg twice daily as fixed-dose product), each in combination with the fixed-dose product, tenofovir DF/emtricitabine (300/200 mg once daily), in 878 antiretroviral treatment-naive subjects. Subjects had a mean age of 36 years (range: 19 to 72), 49% were Caucasian, 18% Black, 9% Asian, 23% Hispanic/Mestizo/mixed race, and 68% were male. The median baseline plasma CD4+ cell count was 204 cells/mm3 (range: 2 to 810 cells/mm3) and the mean baseline plasma HIV-1 RNA level was 4.94 log10 copies/mL (range: 2.60 to 5.88 log10 copies/mL). Treatment response and outcomes through Week 96 are presented in Table 26.
Table 26: Outcomes of Treatment Through Week 96 in Treatment-Naive Adults (Study AI424-138)|
atazanavir |
lopinavir/ritonavirb 400 mg/100 mg (twice daily) with | |
|
Outcome |
96 Weeks |
96 Weeks |
|
Responderc,d,e |
75% |
68% |
|
Virologic failuref |
17% |
19% |
|
Rebound |
8% |
10% |
|
Never suppressed through Week 96 |
9% |
9% |
|
Death |
1% |
1% |
|
Discontinued due to adverse event |
3% |
5% |
|
Discontinued for other reasonsg |
4% |
7% |
|
a As a fixed-dose product: 300 mg tenofovir DF/200 mg emtricitabine once
daily. |
Through 96 weeks of therapy, the proportion of responders among subjects with high viral loads (i.e., baseline HIV-1 RNA ≥100,000 copies/mL) was comparable for the atazanavir with ritonavir (165 of 223 subjects, 74%) and lopinavir/ritonavir (148 of 222 subjects, 67%) arms. At 96 weeks, the median increase from baseline in CD4+ cell count was 261 cells/mm3 for the atazanavir with ritonavir arm and 273 cells/mm3 for the lopinavir/ritonavir arm.
Study AI424-034: Atazanavir once daily compared to efavirenz once daily, each in combination with fixed-dose lamivudine/zidovudine twice daily. Study AI424-034 (NCT00013897) was a randomized, double-blind, multicenter trial comparing atazanavir (400 mg once daily) to efavirenz (600 mg once daily), each in combination with the fixed-dose product of lamivudine/zidovudine (150 mg/300 mg) given twice daily, in 810 antiretroviral treatment-naive subjects. Subjects had a mean age of 34 years (range: 18 to 73), 36% were Hispanic, 33% were Caucasian, and 65% were male. The mean baseline CD4+ cell count was 321 cells/mm3 (range: 64 to 1424 cells/mm3) and the mean baseline plasma HIV-1 RNA level was 4.8 log10 copies/mL (range: 2.2 to 5.9 log10 copies/mL). Treatment response and outcomes through Week 48 are presented in Table 27.
Table 27: Outcomes of Randomized Treatment Through Week 48 in Treatment-Naive Adults (Study AI424-034)|
atazanavir |
efavirenz | |
|
Outcome |
(n=405) |
(n=405) |
|
Respondera |
67% (32%) |
62% (37%) |
|
Virologic failureb |
20% |
21% |
|
Rebound |
17% |
16% |
|
Never suppressed through Week 48 |
3% |
5% |
|
Death |
– |
<1% |
|
Discontinued due to adverse event |
5% |
7% |
|
Discontinued for other reasonsc |
8% |
10% |
|
a Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL (<50
copies/mL) through Week 48. Roche Amplicor® HIV-1 MonitorTM Assay, test
version 1.0 or 1.5 as geographically appropriate. |
Through 48 weeks of therapy, the proportion of responders among subjects with high viral loads (i.e., baseline HIV-1 RNA ≥100,000 copies/mL) was comparable for the atazanavir and efavirenz arms. The mean increase from baseline in CD4+ cell count was 176 cells/mm3 for the atazanavir arm and 160 cells/mm3 for the efavirenz arm.
Study AI424-008: Atazanavir 400 mg once daily compared to atazanavir 600 mg once daily, and compared to nelfinavir 1250 mg twice daily, each in combination with stavudine and lamivudine twice daily. Study AI424-008 (NCT identifier not available) was a 48-week, randomized, multicenter trial, blinded to dose of atazanavir, comparing atazanavir at two dose levels (400 mg and 600 mg once daily) to nelfinavir (1250 mg twice daily), each in combination with stavudine (40 mg) and lamivudine (150 mg) given twice daily, in 467 antiretroviral treatment-naive subjects. Subjects had a mean age of 35 years (range: 18 to 69), 55% were Caucasian, and 63% were male. The mean baseline CD4+ cell count was 295 cells/mm3 (range: 4 to 1003 cells/mm3) and the mean baseline plasma HIV-1 RNA level was 4.7 log10 copies/mL (range: 1.8 to 5.9 log10 copies/mL). Treatment response and outcomes through Week 48 are presented in Table 28.
Table 28: Outcomes of Randomized Treatment Through Week 48 in Treatment-Naive Adults (Study AI424-008)|
atazanavir |
nelfinavir | |
|
Outcome |
(n=181) |
(n=91) |
|
Respondera |
67% (33%) |
59% (38%) |
|
Virologic failureb |
24% |
27% |
|
Rebound |
14% |
14% |
|
Never suppressed through Week 48 |
10% |
13% |
|
Death |
<1% |
– |
|
Discontinued due to adverse event |
1% |
3% |
|
Discontinued for other reasonsc |
7% |
10% |
|
a Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL (<50
copies/mL) through Week 48. Roche Amplicor® HIV-1 MonitorTM Assay, test
version 1.0 or 1.5 as geographically appropriate. |
Through 48 weeks of therapy, the mean increase from baseline in CD4+ cell count was 234 cells/mm3 for the atazanavir 400 mg arm and 211 cells/mm3 for the nelfinavir arm.
14.2 Adult Subjects with Prior Antiretroviral Therapy
Study AI424-045: Atazanavir once daily with ritonavir once daily compared to atazanavir once daily and saquinavir (soft gelatin capsules) once daily, and compared to lopinavir/ritonavir twice daily, each in combination with tenofovir DF and one NRTI. Study AI424-045 (NCT00035932): was a randomized, multicenter trial comparing atazanavir (300 mg once daily) with ritonavir (100 mg once daily) to atazanavir (400 mg once daily) with saquinavir soft gelatin capsules (1200 mg once daily), and to lopinavir/ritonavir (400/100 mg twice daily as fixed-dose product), each in combination with tenofovir DF and one NRTI, in 347 (of 358 randomized) subjects who experienced virologic failure on highly active antiretroviral therapy regimens containing PIs, NNRTIs, and NRTIs. The mean time of prior exposure to antiretrovirals was 139 weeks for PIs, 85 weeks for NNRTIs, and 283 weeks for NRTIs. The mean age was 41 years (range: 24 to 74); 60% were Caucasian, and 78% were male. The mean baseline CD4+ cell count was 338 cells/mm3 (range: 14 to 1543 cells/mm3) and the mean baseline plasma HIV-1 RNA level was 4.4 log10 copies/mL (range: 2.6 to 5.88 log10 copies/mL).
Treatment outcomes through Week 48 for the atazanavir with ritonavir and lopinavir/ritonavir treatment arms are presented in Table 29. Atazanavir with ritonavir and lopinavir/ritonavir were similar for the primary efficacy outcome measure of time-averaged difference in change from baseline in HIV-1 RNA level. Study AI424-045 was not large enough to reach a definitive conclusion that atazanavir with ritonavir and lopinavir/ritonavir are equivalent on the secondary efficacy outcome measure of proportions below the HIV-1 RNA lower limit of quantification [see Microbiology, Tables 24 and 25 (12.4)].
Table 29: Outcomes of Treatment Through Week 48 in Study AI424-045 (Subjects with Prior Antiretroviral Experience)|
atazanavir |
lopinavir/ritonavir |
Difference****a | |
|
Outcome |
(n=119) |
(n=118) |
(CI) |
|
HIV-1 RNA Change from |
−1.58 |
−1.70 |
+0.12c |
|
CD4+ Change from Baseline (cells/mm3)e |
116 |
123 |
−7 (−67, 52) |
|
Percent of Subjects Respondinge | |||
|
HIV-1 RNA <400 copies/mLc |
55% |
57% |
-2.2% |
|
HIV-1 RNA <50 copies/mLc |
38% |
45% |
−7.1% |
|
a Time-averaged difference through Week 48 for HIV-1 RNA; Week 48 difference
in HIV-1 RNA percentages and CD4+ mean changes, atazanavir with ritonavir vs.
lopinavir/ritonavir; CI = 97.5% confidence interval for change in HIV-1 RNA;
95% confidence interval otherwise. |
No subjects in the atazanavir with ritonavir treatment arm and three subjects in the lopinavir/ritonavir treatment arm experienced a new-onset CDC Category C event during the study.
In Study AI424-045, the mean change from baseline in plasma HIV-1 RNA for atazanavir 400 mg with saquinavir (n=115) was −1.55 log10 copies/mL, and the time-averaged difference in change in HIV-1 RNA levels versus lopinavir/ritonavir was 0.33. The corresponding mean increase in CD4+ cell count was 72 cells/mm3. Through 48 weeks of treatment, the proportion of subjects in this treatment arm with plasma HIV-1 RNA <400 (<50) copies/mL was 38% (26%). In this study, coadministration of atazanavir and saquinavir did not provide adequate efficacy [see Drug Interactions (7)].
Study AI424-045 also compared changes from baseline in lipid values. [See Adverse Reactions (6.1).]
Study AI424-043 (NCT00028301): Study AI424-043 was a randomized, open-label, multicenter trial comparing atazanavir (400 mg once daily) to lopinavir/ritonavir (400/100 mg twice daily as fixed-dose product), each in combination with two NRTIs, in 300 subjects who experienced virologic failure to only one prior PI-containing regimen. Through 48 weeks, the proportion of subjects with plasma HIV-1 RNA <400 (<50) copies/mL was 49% (35%) for subjects randomized to atazanavir (n=144) and 69% (53%) for subjects randomized to lopinavir/ritonavir (n=146). The mean change from baseline was −1.59 log10 copies/mL in the atazanavir treatment arm and −2.02 log10 copies/mL in the lopinavir/ritonavir arm. Based on the results of this study, atazanavir without ritonavir was inferior to lopinavir/ritonavir in PI-experienced subjects with prior virologic failure and is not recommended for such patients.
14.3 Pediatric Subjects
Pediatric Trials with Atazanavir Capsules
Study AI424-040; PACTG 1020A (NCT00006604): Assessment of the pharmacokinetics, safety, tolerability, and virologic response of atazanavir capsules was based on data from this open-label, multicenter clinical trial which included subjects from 6 years to 21 years of age. In this study, 105 subjects (43 antiretroviral-naive and 62 antiretroviral-experienced) received once daily atazanavir capsule formulation, with or without ritonavir, in combination with two NRTIs.
One-hundred five (105) subjects (6 to less than 18 years of age) treated with the atazanavir capsule formulation, with or without ritonavir, were evaluated. Using an intent-to-treat (ITT) analysis, the overall proportions of antiretroviral-naive and -experienced subjects with HIV-1 RNA <400 copies/mL at Week 96 were 51% (22/43) and 34% (21/62), respectively. The overall proportions of antiretroviral-naive and -experienced subjects with HIV-1 RNA <50 copies/mL at Week 96 were 47% (20/43) and 24% (15/62), respectively. The median increase from baseline in absolute CD4 count at 96 weeks of therapy was 335 cells/mm3 in antiretroviral-naive subjects and 220 cells/mm3 in antiretroviral-experienced subjects.
Information For Patients Section
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Atazanavir is not a cure for HIV-1 infection. Advise patients to remain under the care of a healthcare provider while using atazanavir.
Cardiac Conduction Abnormalities
Inform patients that atazanavir may produce changes in the electrocardiogram (e.g., PR prolongation). Tell patients to consult their healthcare provider if they are experiencing symptoms such as dizziness or lightheadedness [see Warnings and Precautions (5.1)].
Severe Skin Reaction
Inform patients that there have been reports of severe skin reactions (e.g., Stevens-Johnson syndrome, erythema multiforme, and toxic skin eruptions) with atazanavir use. Advise patients that if signs or symptoms of severe skin reactions or hypersensitivity reactions develop, they must discontinue atazanavir and seek medical evaluation immediately [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Hyperbilirubinemia
Inform patients that asymptomatic elevations in indirect bilirubin have occurred in patients receiving atazanavir. This may be accompanied by yellowing of the skin or whites of the eyes and alternative antiretroviral therapy may be considered if the patient has cosmetic concerns [see Warnings and Precautions (5.8)].
Chronic Kidney Disease
Inform patients that treatment with atazanavir may lead to the development of chronic kidney disease, and to maintain adequate hydration while taking atazanavir [see Warnings and Precautions (5.5)].
Nephrolithiasis and Cholelithiasis
Inform patients that kidney stones and/or gallstones have been reported with atazanavir use. Some patients with kidney stones and/or gallstones required hospitalization for additional management, and some had complications. Discontinuation of atazanavir may be necessary as part of the medical management of these adverse events [see Warnings and Precautions (5.6)].
Drug Interactions
Atazanavir may lead to significant interaction with some drugs; therefore, advise patients to report the use of any other prescription, nonprescription medication, or herbal products, particularly St. John’s wort, to their healthcare provider prior to use [see Contraindications (4), Warnings and Precautions (5.7)].
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.10)].
Fat Redistribution
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy including protease inhibitors and that the cause and long-term health effects of these conditions are not known at this time [see Warnings and Precautions (5.11)].
Dosing Instructions
Advise patients to take atazanavir with food every day and take other concomitant antiretroviral therapy as prescribed. Atazanavir must always be used in combination with other antiretroviral drugs. Advise patients that they should not alter the dose or discontinue therapy without consulting with their healthcare provider. Tell patients if a dose of atazanavir is missed, they should take the dose as soon as possible and then return to their normal schedule; however, if a dose is skipped the patient should not double the next dose.
Pregnancy
Inform pregnant patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in pregnant patients exposed to atazanavir during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk. Atazanavir can also be passed to the baby in breast milk, and it is not known whether it could harm the baby [see Use in Specific Populations (8.2)].
Manufactured for: Northstar Rx LLC
Memphis, TN 38141.
Manufactured by: Aurobindo Pharma Limited
Unit-VII (SEZ)
Mahabubnagar (Dt)-509302
India.
M.L.No.: 22/MN/AP/2009/F/R
Revised: 01/2024
