SINUS PAIN AND CONGESTION
CVS 44-466C-SCH
c878c7aa-c591-4bae-a1d1-842eaf27c5b1
HUMAN OTC DRUG LABEL
Sep 18, 2025
CVS Pharmacy
DUNS: 062312574
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acetaminophen and Phenylephrine HCl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
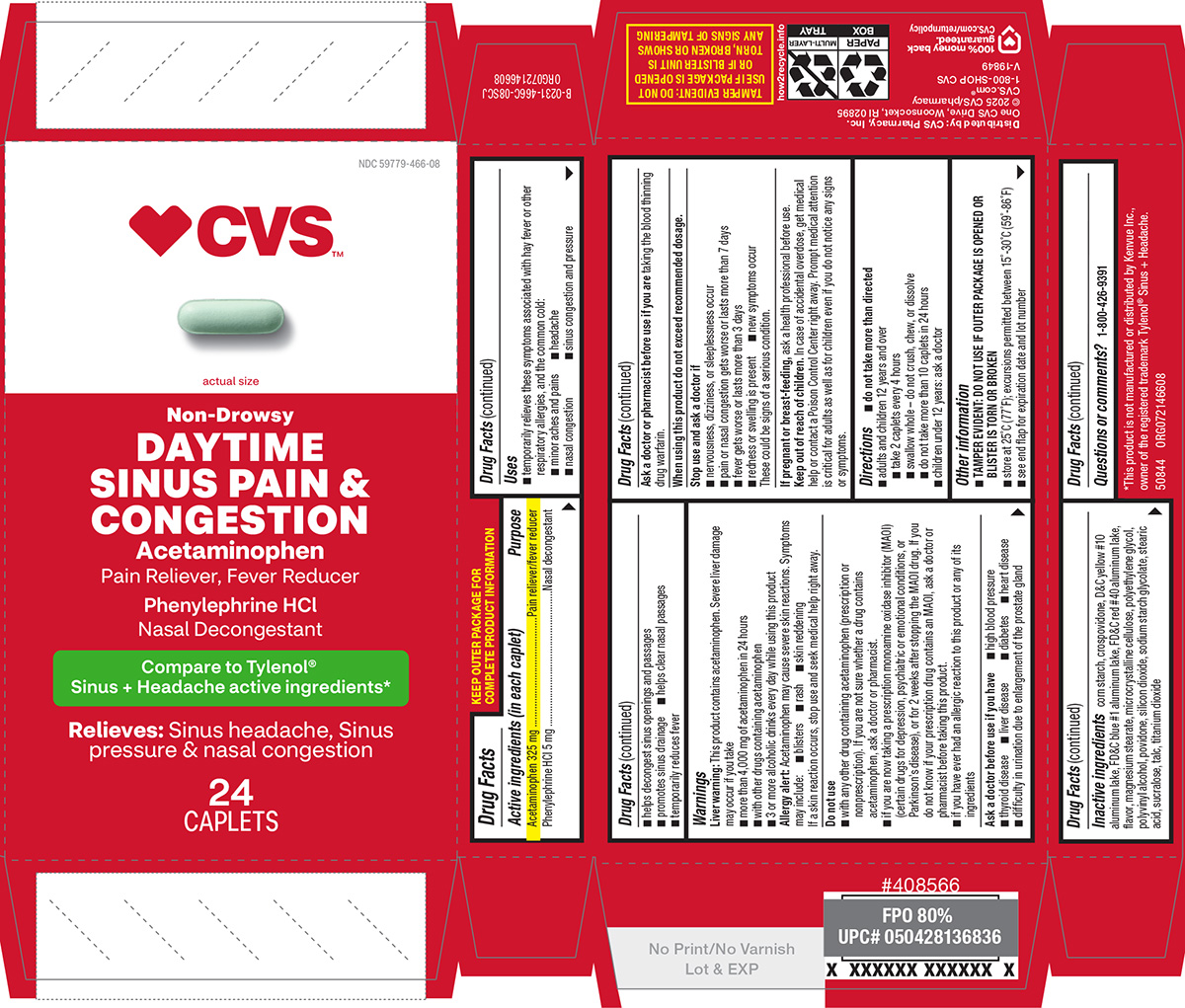
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
♥CVS**™**
actual size****
****** Non-Drowsy**
DAYTIME****
****** SINUS PAIN &******
****** CONGESTION**
Acetaminophen****
****** Pain Reliever, Fever Reducer******
****** Phenylephrine HCl******
****** Nasal Decongestant******
Compare to Tylenol® Sinus + Headache
active ingredients*
Relieves:
Sinus headache,
Sinus pressure &
Nasal congestion
24CAPLETS
TAMPER EVIDENT: DO NOT USE IF
** PACKAGE IS OPENED OR IF BLISTER**
** UNIT IS TORN, BROKEN OR SHOWS**
** ANY SIGNS OF TAMPERING**
*This product is not manufactured or distributed by Kenvue Inc.,
owner of the registered trademark Tylenol® Sinus + Headache.
50844 ORG072146608
Distributed by: CVS Pharmacy, Inc.
****One CVS Drive, Woonsocket, RI 02895
© 2025 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
V-19849
100% money back
guaranteed.
CVS.com/returnpolicy
CVS 44-466C ORG0721
INDICATIONS & USAGE SECTION
Uses
- temporarily relieves these symptoms associated with hay fever or other respiratory allergies, and the common cold:
- minor aches and pains
- headache
- nasal congestion
- sinus congestion and pressure
- helps decongest sinus openings and passages
- promotes sinus drainage
- helps clear nasal passages
- temporarily reduces fever
DOSAGE & ADMINISTRATION SECTION
Directions
*do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 4 hours
- swallow whole - do not crush, chew, or dissolve
- do not take more than 10 caplets in 24 hours
- children under 12 years: ask a doctor
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (in each caplet)
Acetaminophen 325 mg
Phenylephrine HCl 5 mg
OTC - PURPOSE SECTION
Purpose
Pain reliever/fever reducer
Nasal decongestant
WARNINGS SECTION
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- heart disease
- diabetes
- thyroid disease
- high blood pressure
Ask a doctor or pharmacist before use if you are
taking the blood thinning drug warfarin.
When using this product
do not exceed recommended dosage.
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain or nasal congestion gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
STORAGE AND HANDLING SECTION
Other information
*TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
INACTIVE INGREDIENT SECTION
Inactive ingredients
corn starch, crospovidone, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C red #40 aluminum lake, flavor, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, sucralose, talc, titanium dioxide
OTC - QUESTIONS SECTION
Questions or comments?
1-800-426-9391
