Oxaliplatin
These highlights do not include all the information needed to use OXALIPLATIN INJECTION, USP safely and effectively. See full prescribing information for OXALIPLATIN INJECTION, USP.OXALIPLATIN Injection, for intravenous use Initial U.S. Approval: 2002
e12e2850-4980-48de-af58-15e559fb921f
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
Fresenius Kabi USA, LLC
DUNS: 608775388
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Oxaliplatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL PRINCIPAL DISPLAY PANEL- 50 mg per 10 mL- Vial Carton Panel
** NDC** 63323-750-10 775010
Oxaliplatin Injection, USP
50 mg per 10 mL
** (5 mg per mL)**
For intravenous use only.
MUST BE DILUTED WITH 5% DEXTROSE INJECTION BEFORE USE.
** Preservative free.**
** Cytotoxic agent.**
Do not mix or add to sodium chloride/chloride-containing solutions.
Single Dose Vial Rx only

Boxed Warning section
WARNING: HYPERSENSITIVITY REACTIONS, INCLUDING ANAPHYLAXIS
See full prescribing information for complete boxed warning.
Serious and fatal hypersensitivity adverse reactions, including anaphylaxis, can occur with OXALIPLATIN INJECTION, USP within minutes of administration and during any cycle. OXALIPLATIN INJECTION, USP is contraindicated in patients with hypersensitivity reactions to oxaliplatin and other platinum-based drugs. Immediately and permanently discontinue OXALIPLATIN INJECTION, USP for hypersensitivity reactions and administer appropriate treatment. (4, 5.1)
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Oxaliplatin Injection, in combination with infusional fluorouracil and leucovorin, is indicated for:
•
adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor.
•
treatment of advanced colorectal cancer.
Oxaliplatin Injection is a platinum-based drug used in combination with infusional fluorouracil and leucovorin, which is indicated for:
•
adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor. (1)
•
treatment of advanced colorectal cancer. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Oxaliplatin is contraindicated in patients with a history of a hypersensitivity reaction to oxaliplatin or other platinum-based drugs. Reactions have included anaphylaxis [see Warnings and Precautions (5.1)].
•
History of hypersensitivity reaction to oxaliplatin or other platinum-based drugs. (4, 5.1)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious and fatal hypersensitivity reactions, including anaphylaxis, can occur with Oxaliplatin within minutes of administration and during any cycle. Grade 3-4 hypersensitivity reactions, including anaphylaxis, occurred in 2% to 3% of patients with colon cancer who received Oxaliplatin. Hypersensitivity reactions, including rash, urticaria, erythema, pruritus, and, rarely, bronchospasm and hypotension, were similar in nature and severity to those reported with other platinum-based drugs.
Oxaliplatin is contraindicated in patients with hypersensitivity reactions to platinum-based drugs [see Contraindications (4)]. Immediately and permanently discontinue Oxaliplatin for hypersensitivity reactions and administer appropriate treatment for management of hypersensitivity reactions.
5.2 Peripheral Sensory Neuropathy
Oxaliplatin can cause acute and delayed neuropathy. Reduce the dose or permanently discontinue Oxaliplatin for persistent neurosensory reactions based on the severity of the adverse reaction [see Dosage and Administration (2.3)].
Acute Neuropathy
Acute neuropathy typically presents as a reversible, primarily peripheral sensory neuropathy that occurs within hours or 2 days following a dose, resolves within 14 days, and frequently recurs with further dosing. The symptoms can be precipitated or exacerbated by exposure to cold temperature or cold objects and they usually present as transient paresthesia, dysesthesia and hypoesthesia in the hands, feet, perioral area, or throat. Jaw spasm, abnormal tongue sensation, dysarthria, eye pain, and a feeling of chest pressure have also been observed. The acute, reversible pattern of sensory neuropathy was observed in about 56% of patients who received Oxaliplatin Injection with fluorouracil/leucovorin. In any individual cycle, acute neuropathy occurred in approximately 30% of patients. For grade 3 peripheral sensory neuropathy, the median time to onset was 9 cycles for adjuvant treatment and 6 cycles for previously treated advanced colorectal cancer.
An acute syndrome of pharyngolaryngeal dysesthesia occurred in 1% to 2% (grade 3 to 4) of patients previously untreated for advanced colorectal cancer. Subjective sensations of dysphagia or dyspnea, without any laryngospasm or bronchospasm (no stridor or wheezing) occurred in patients previously treated for advanced colorectal cancer.
Avoid topical application of ice for mucositis prophylaxis or other conditions, because cold temperature can exacerbate acute neurological symptoms.
Delayed Neuropathy
Delayed neuropathy typically presents as a persistent (greater than 14 days), primarily peripheral sensory neuropathy that is usually characterized by paresthesias, dysesthesias, and hypoesthesias, but may also include deficits in proprioception that can interfere with daily activities (e.g., writing, buttoning, swallowing, and difficulty walking from impaired proprioception). These forms of neuropathy occurred in 48% of patients receiving Oxaliplatin. Delayed neuropathy can occur without any prior acute neuropathy. Most patients (80%) who developed grade 3 persistent neuropathy progressed from prior grade 1 or 2 reactions. These symptoms may improve in some patients upon discontinuation of Oxaliplatin.
Adjuvant treatment
In the adjuvant treatment trial, neuropathy was graded using NCI CTC, version 1 as summarized in Table 3.
Table 3: Grading for Neuropathy in Adjuvant Treatment Trial|
Grade |
Definition |
|
0 |
No change or none |
|
1 |
Mild paresthesias, loss of deep tendon reflexes |
|
2 |
Mild or moderate objective sensory loss, moderate paresthesias |
|
3 |
Severe objective sensory loss or paresthesias that interfere with function |
|
4 |
Not applicable |
Peripheral sensory neuropathy occurred in 92% of patients (all grades), including 13% of patients (grade 3) who received Oxaliplatin with fluorouracil/leucovorin. At the 28-day follow-up after the last treatment cycle, 60% of patients had any grade (grade 1=40%, grade 2=16%, grade 3=5%) peripheral sensory neuropathy, decreasing to 39% at 6 months of follow-up (grade 1=31%, grade 2=7%, grade 3=1%) and 21% at 18 months of follow-up (grade 1=17%, grade 2=3%, grade 3=1%).
Advanced colorectal cancer
In the advanced colorectal cancer trials, neuropathy was graded using the neurotoxicity scale summarized in Table 4.
Table 4: Grading for Neuropathy in Advanced Colorectal Cancer Trials|
Grade |
Definition |
|
1 |
Resolved and did not interfere with functioning |
|
2 |
Interfered with function but not daily activities |
|
3 |
Pain or functional impairment that interfered with daily activities |
|
4 |
Persistent impairment that is disabling or life-threatening |
Neuropathy occurred in 82% (all grades) of patients previously untreated for advanced colorectal cancer, including 19% grade 3-4; and in 74% (all grades) of patients previously treated for advanced colorectal cancer, including 7% grade 3-4.
5.3 Severe Myelosuppression
Grade 3 or 4 neutropenia occurred in 41% to 44% of patients with colorectal cancer who received Oxaliplatin with fluorouracil/leucovorin. Sepsis, neutropenic sepsis and septic shock, including fatal outcomes, occurred in patients who received Oxaliplatin [see Adverse Reactions (6.1, 6.2)].
Grade 3 or 4 thrombocytopenia occurred in 2% to 5% of patients with colorectal cancer who received Oxaliplatin with fluorouracil/leucovorin.
Monitor complete blood cell count at baseline, before each subsequent cycle and as clinically indicated. Delay Oxaliplatin until neutrophils are greater than or equal to 1.5 × 109/L and platelets are greater than or equal to 75 × 109/L. Withhold Oxaliplatin for sepsis or septic shock. Dose reduce Oxaliplatin after recovery from grade 4 neutropenia, febrile neutropenia or grade 3-4 thrombocytopenia as recommended [see Dosage and Administration (2.2)].
5.4 Posterior Reversible Encephalopathy Syndrome
PRES occurred in less than 0.1% of patients across clinical trials [see Adverse Reactions (6.1)]. Signs and symptoms of PRES can include headache, altered mental functioning, seizures, abnormal vision from blurriness to blindness, associated or not with hypertension. Confirm the diagnosis of PRES with magnetic resonance imaging. Permanently discontinue Oxaliplatin in patients who develop PRES.
5.5 Pulmonary Toxicity
Oxaliplatin has been associated with pulmonary fibrosis (less than 1% of patients), which may be fatal [see Adverse Reactions (6.1)].
In the adjuvant treatment trial, the combined incidence of cough and dyspnea was 7.4% (any grade), including less than 1% (grade 3) in the Oxaliplatin arm. One patient died from eosinophilic pneumonia in the Oxaliplatin arm.
In the previously untreated advanced colorectal cancer trial, the combined incidence of cough, dyspnea and hypoxia was 43% (any grade), including 7% (grade 3-4) in the Oxaliplatin with fluorouracil/leucovorin arm.
In case of unexplained respiratory symptoms, such as non-productive cough, dyspnea, crackles, or radiological pulmonary infiltrates, withhold Oxaliplatin until further pulmonary investigation excludes interstitial lung disease or pulmonary fibrosis. Permanently discontinue Oxaliplatin for confirmed interstitial lung disease or pulmonary fibrosis.
5.6 Hepatotoxicity
In the adjuvant treatment trial, increased transaminases (57% vs 34%) and alkaline phosphatase (42% vs 20%) occurred more commonly in the Oxaliplatin arm than in the fluorouracil/leucovorin arm [see Adverse Reactions (6.1)]. The incidence of increased bilirubin was similar on both arms. Changes noted on liver biopsies include: peliosis, nodular regenerative hyperplasia or sinusoidal alterations, perisinusoidal fibrosis, and veno-occlusive lesions. Consider evaluating patients who develop abnormal liver tests or portal hypertension which cannot be explained by liver metastases, for hepatic vascular disorders. Monitor liver function tests at baseline, before each subsequent cycle and as clinically indicated.
5.7 QT Interval Prolongation and Ventricular Arrhythmias
QT prolongation and ventricular arrhythmias, including fatal torsade de pointes, have been reported with Oxaliplatin [see Adverse Reactions (6.2)].
Avoid Oxaliplatin in patients with congenital long QT syndrome. Monitor electrocardiograms (ECG) in patients with congestive heart failure, bradyarrhythmias, and electrolyte abnormalities and in patients taking drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics [see Drug Interactions (7.1)]. Monitor and correct electrolyte abnormalities prior to initiating Oxaliplatin and periodically during treatment.
5.8 Rhabdomyolysis
Rhabdomyolysis, including fatal cases, has been reported with Oxaliplatin [see Adverse Reactions (6.2)]. Permanently discontinue Oxaliplatin for any signs or symptoms of rhabdomyolysis.
5.9 Hemorrhage
The incidence of hemorrhage in clinical trials was higher on the Oxaliplatin combination arm compared to the fluorouracil/leucovorin arm. These reactions included gastrointestinal bleeding, hematuria, and epistaxis. In the adjuvant treatment trial, 2 patients died from intracerebral hemorrhage [see Adverse Reactions (6.1)].
Prolonged prothrombin time and INR occasionally associated with hemorrhage have been reported in patients who received Oxaliplatin with fluorouracil/leucovorin while on anticoagulants [see Adverse Reactions (6.2)]. Increase frequency of monitoring in patients who are receiving Oxaliplatin with fluorouracil/leucovorin and oral anticoagulants [see Drug Interactions (7.3)].
Thrombocytopenia and immune-mediated thrombocytopenia have been observed with Oxaliplatin. Rapid onset of thrombocytopenia and greater risk of bleeding have been observed in immune-mediated thrombocytopenia. In this case, consider discontinuing Oxaliplatin.
5.10 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, Oxaliplatin can cause fetal harm when administered to a pregnant woman. The available human data do not establish the presence or absence of major birth defects or miscarriage related to the use of Oxaliplatin. Reproductive toxicity studies demonstrated adverse effects on embryo-fetal development in rats at maternal doses that were below the recommended human dose based on body surface area.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Oxaliplatin and for at least 9 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Oxaliplatin and for 6 months after the final dose [see Use in Specific Populations (8.1, 8.3)].
•
Peripheral Sensory Neuropathy: Acute and delayed neuropathy can occur. Avoid topical application of ice. Reduce the dose or permanently discontinue Oxaliplatin Injection as recommended. (5.2)
•
Severe Myelosuppression: Delay Oxaliplatin Injection until neutrophils are greater than or equal to 1.5 × 109/L and platelets are greater than or equal to 75 × 109/L. Withhold Oxaliplatin Injection for sepsis or septic shock. Dose reduce after recovery from grade 4 neutropenia, febrile neutropenia, or grade 3-4 thrombocytopenia as recommended. (5.3)
•
Posterior Reversible Encephalopathy Syndrome (PRES): Permanently discontinue Oxaliplatin Injection in patients who develop PRES. (5.4)
•
Pulmonary Toxicity: Withhold Oxaliplatin Injection until investigation excludes interstitial lung disease or pulmonary fibrosis. (5.5)
•
Hepatotoxicity: Monitor liver function tests at baseline, before each subsequent cycle, and as clinically indicated. (5.6)
•
QT Interval Prolongation: Avoid in patients with congenital long QT syndrome. Monitor electrocardiograms in patients with congestive heart failure, bradyarrhythmias, and electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Correct electrolyte abnormalities prior to initiating Oxaliplatin Injection and periodically during treatment. (5.7)
•
Rhabdomyolysis: Permanently discontinue Oxaliplatin Injection if rhabdomyolysis occurs. (5.8)
•
Hemorrhage: Increase frequency of monitoring in patients who are receiving Oxaliplatin Injection with fluorouracil/leucovorin and oral anticoagulants (5.9)
•
Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus. Advise males and females of reproductive potential to use an effective method of contraception. (5.10, 8.1, 8.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
•
Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
•
Peripheral Sensory Neuropathy [see Warnings and Precautions (5.2)]
•
Severe Myelosuppression [see Warnings and Precautions (5.3)]
•
Reversible Posterior Leukoencephalopathy Syndrome [see Warnings and Precautions (5.4)]
•
Pulmonary Toxicity [see Warnings and Precautions (5.5)]
•
Hepatotoxicity [see Warnings and Precautions (5.6)]
•
QT Interval Prolongation and Ventricular Arrhythmias [see Warnings and Precautions (5.7)]
•
Rhabdomyolysis [see Warnings and Precautions (5.8)]
•
Hemorrhage [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
More than 1,100 patients with stage II or III colon cancer and more than 4,000 patients with advanced colorectal cancer were treated in trials with Oxaliplatin. The most common adverse reactions in patients with stage II or III colon cancer receiving adjuvant treatment were peripheral sensory neuropathy, neutropenia, thrombocytopenia, anemia, nausea, increase in transaminases and alkaline phosphatase, diarrhea, emesis, fatigue and stomatitis. The most common adverse reactions in previously untreated and treated patients with advanced colorectal cancer were peripheral sensory neuropathies, fatigue, neutropenia, nausea, emesis, and diarrhea.
Adjuvant Treatment
The safety of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in patients with stage II or III colon cancer, who had undergone complete resection of the primary tumor in the adjuvant treatment trial [see Clinical Studies (14.1)].
Fatal adverse reactions in patients who received Oxaliplatin in the combination arm included sepsis/neutropenic sepsis (n=3), intracerebral hemorrhage (n=2) and eosinophilic pneumonia (n=1).
Thromboembolic events occurred in 6% (grade 3-4, 1.2%) of patients in the Oxaliplatin arm.
Grade 3 or 4 adverse reactions occurred in 70% of patients in the Oxaliplatin arm. Grade 3-4 gastrointestinal bleeding occurred in 0.2% of patients. Febrile neutropenia occurred in 0.7% and documented infection with concomitant grade 3-4 neutropenia occurred in 1.1%.
Discontinuation due to an adverse reaction occurred in 15% of the patients in the Oxaliplatin arm.
Tables 5, 6, and 7 summarize the adverse reactions reported in patients with colon cancer receiving adjuvant treatment.
Table 5: Adverse Reactions Reported in Patients with Colon Cancer Receiving Adjuvant Treatment (greater than or equal to 5% of all patients and with greater than or equal to 1% grade 3-4)
| ||||
|
† Includes thrombosis related to the catheter | ||||
|
Adverse Reaction* |
Oxaliplatin + FU/LV N=1108 |
FU/LV N=1111 | ||
|
All Grades (%) |
Grade 3-4 (%) |
All Grades (%) |
Grade 3-4 (%) | |
|
Neurology | ||||
|
Peripheral Sensory Neuropathy |
92 |
12 |
16 |
<1 |
|
Gastrointestinal | ||||
|
Nausea |
74 |
5 |
61 |
2 |
|
Diarrhea |
56 |
11 |
48 |
7 |
|
Vomiting |
47 |
6 |
24 |
1 |
|
Stomatitis |
42 |
3 |
40 |
2 |
|
Anorexia |
13 |
1 |
8 |
<1 |
|
Constitutional Symptoms/Pain | ||||
|
Fatigue |
44 |
4 |
38 |
1 |
|
Abdominal Pain |
18 |
1 |
17 |
2 |
|
Dermatology/Skin | ||||
|
Skin Disorder |
32 |
2 |
36 |
2 |
|
Injection Site Reaction† |
11 |
3 |
10 |
3 |
|
Fever/Infection | ||||
|
Fever |
27 |
1 |
12 |
1 |
|
Infection |
25 |
4 |
25 |
3 |
|
Allergy/Immunology | ||||
|
Allergic Reaction |
10 |
3 |
2 |
<1 |
| ||
|
† No complete alopecia was reported. | ||
|
Adverse Reaction* |
Oxaliplatin + FU/LV N=1108 |
FU/LV |
|
All Grades (%) |
All Grades | |
|
Dermatology/Skin | ||
|
Alopecia† |
30 |
28 |
|
Gastrointestinal | ||
|
Constipation |
22 |
19 |
|
Taste Perversion |
12 |
8 |
|
Dyspepsia |
8 |
5 |
|
Constitutional Symptoms/Pain/Ocular/Visual | ||
|
Epistaxis |
16 |
12 |
|
Weight Increase |
10 |
10 |
|
Conjunctivitis |
9 |
15 |
|
Headache |
7 |
5 |
|
Dyspnea |
5 |
3 |
|
Pain |
5 |
5 |
|
Abnormal Lacrimation |
4 |
12 |
|
Neurology | ||
|
Sensory Disturbance |
8 |
1 |
|
Allergy/Immunology | ||
|
Rhinitis |
6 |
8 |
|
Laboratory-Related Adverse Reaction |
Oxaliplatin with FU/LV |
FU/LV | ||
|
All Grades |
Grades 3-4 |
All Grades |
Grades 3-4 | |
|
Hematology | ||||
|
Neutropenia |
79 |
41 |
40 |
5 |
|
Thrombocytopenia |
77 |
2 |
19 |
<1 |
|
Anemia |
76 |
1 |
67 |
<1 |
|
Hepatic | ||||
|
Increased Transaminases |
57 |
2 |
34 |
1 |
|
Increased Alkaline Phosphatase |
42 |
<1 |
20 |
<1 |
|
Hyperbilirubinemia |
20 |
4 |
20 |
5 |
Previously Untreated Advanced Colorectal Cancer
The safety of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a randomized trial of patients with previously untreated advanced colorectal cancer [see Clinical Studies (14.2)]. The adverse reaction profile in this trial was similar to that seen in other trials.
Tables 8, 9, and 10 summarize the adverse reactions reported in the previously untreated advanced colorectal cancer trial.
Table 8: Adverse Reactions Reported in Patients in the Previously Untreated Advanced Colorectal Cancer Clinical Trial (greater than or equal to 5% of all patients and with greater than or equal to 1% grade 3-4)
| ||||||
|
† Not otherwise specified | ||||||
|
‡ Absolute neutrophil count | ||||||
|
Adverse Reaction* |
Oxaliplatin + FU/LV N=259 |
Irinotecan + FU/LV N=256 |
Oxaliplatin + | |||
|
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 | |
|
Neurology | ||||||
|
Neuropathy |
82 |
19 |
18 |
2 |
69 |
7 |
|
Paresthesias |
77 |
18 |
16 |
2 |
62 |
6 |
|
Pharyngo-laryngeal |
38 |
2 |
1 |
0 |
28 |
1 |
|
Neuro-sensory |
12 |
1 |
2 |
0 |
9 |
1 |
|
Neuro NOS† |
1 |
0 |
1 |
0 |
1 |
0 |
|
Gastrointestinal | ||||||
|
Nausea |
71 |
6 |
67 |
15 |
83 |
19 |
|
Diarrhea |
56 |
12 |
65 |
29 |
76 |
25 |
|
Vomiting |
41 |
4 |
43 |
13 |
64 |
23 |
|
Stomatitis |
38 |
0 |
25 |
1 |
19 |
1 |
|
Anorexia |
35 |
2 |
25 |
4 |
27 |
5 |
|
Constipation |
32 |
4 |
27 |
2 |
21 |
2 |
|
Diarrhea-colostomy |
13 |
2 |
16 |
7 |
16 |
3 |
|
Gastrointestinal NOS† |
5 |
2 |
4 |
2 |
3 |
2 |
|
Constitutional Symptoms/Pain/Ocular/Visual | ||||||
|
Fatigue |
70 |
7 |
58 |
11 |
66 |
16 |
|
Abdominal Pain |
29 |
8 |
31 |
7 |
39 |
10 |
|
Myalgia |
14 |
2 |
6 |
0 |
9 |
2 |
|
Pain |
7 |
1 |
5 |
1 |
6 |
1 |
|
Abnormal Vision |
5 |
0 |
2 |
1 |
6 |
1 |
|
Neuralgia |
5 |
0 |
0 |
0 |
2 |
1 |
|
Pulmonary | ||||||
|
Cough |
35 |
1 |
25 |
2 |
17 |
1 |
|
Dyspnea |
18 |
7 |
14 |
3 |
11 |
2 |
|
Hiccups |
5 |
1 |
2 |
0 |
3 |
2 |
|
Hepatic/Metabolic/Laboratory/Renal | ||||||
|
Hyperglycemia |
14 |
2 |
11 |
3 |
12 |
3 |
|
Hypokalemia |
11 |
3 |
7 |
4 |
6 |
2 |
|
Dehydration |
9 |
5 |
16 |
11 |
14 |
7 |
|
Hypoalbuminemia |
8 |
0 |
5 |
2 |
9 |
1 |
|
Hyponatremia |
8 |
2 |
7 |
4 |
4 |
1 |
|
Urinary Frequency |
5 |
1 |
2 |
1 |
3 |
1 |
|
Hematology/Infection | ||||||
|
Infection Normal ANC‡ |
10 |
4 |
5 |
1 |
7 |
2 |
|
Infection Low ANC‡ |
8 |
8 |
12 |
11 |
9 |
8 |
|
Lymphopenia |
6 |
2 |
4 |
1 |
5 |
2 |
|
Febrile Neutropenia |
4 |
4 |
15 |
14 |
12 |
11 |
|
Dermatology/Skin | ||||||
|
Hand/Foot Syndrome |
7 |
1 |
2 |
1 |
1 |
0 |
|
Injection Site Reaction |
6 |
0 |
1 |
0 |
4 |
1 |
|
Cardiovascular | ||||||
|
Thrombosis |
6 |
5 |
6 |
6 |
3 |
3 |
|
Hypotension |
5 |
3 |
6 |
3 |
4 |
3 |
| |||
|
† No complete alopecia was reported. | |||
|
‡ Absolute neutrophil count | |||
|
Adverse Reaction* |
Oxaliplatin + 5-FU/LV N=259 |
Irinotecan + 5-FU/LV N=256 |
Oxaliplatin + |
|
All Grades (%) |
All Grades (%) |
All Grades | |
|
Dermatology/Skin | |||
|
Alopecia† |
38 |
44 |
67 |
|
Flushing |
7 |
2 |
5 |
|
Pruritus |
6 |
4 |
2 |
|
Dry Skin |
6 |
2 |
5 |
|
Hematology/Infection | |||
|
Fever Normal ANC‡ |
16 |
9 |
9 |
|
Cardiovascular | |||
|
Edema |
15 |
13 |
10 |
|
Gastrointestinal | |||
|
Taste Perversion |
14 |
6 |
8 |
|
Dyspepsia |
12 |
7 |
5 |
|
Flatulence |
9 |
6 |
5 |
|
Mouth Dryness |
5 |
2 |
3 |
|
Constitutional Symptoms/Pain/Ocular/Visual | |||
|
Headache |
13 |
6 |
9 |
|
Weight Loss |
11 |
9 |
11 |
|
Epistaxis |
10 |
2 |
2 |
|
Tearing |
9 |
1 |
2 |
|
Rigors |
8 |
2 |
7 |
|
Dysphasia |
5 |
3 |
3 |
|
Sweating |
5 |
6 |
12 |
|
Arthralgia |
5 |
5 |
8 |
|
Neurology | |||
|
Insomnia |
13 |
9 |
11 |
|
Depression |
9 |
5 |
7 |
|
Dizziness |
8 |
6 |
10 |
|
Anxiety |
5 |
2 |
6 |
|
Allergy/Immunology | |||
|
Rash |
11 |
4 |
7 |
|
Rhinitis Allergic |
10 |
6 |
6 |
|
Hepatic/Metabolic/Laboratory/Renal | |||
|
Hypocalcemia |
7 |
5 |
4 |
|
Elevated Creatinine |
4 |
4 |
5 |
Clinically relevant adverse reactions that occurred in greater than or equal to 2% and less than 5% of the patients in the Oxaliplatin and fluorouracil/leucovorin combination arm (listed in decreasing order of frequency) were: metabolic, pneumonitis, catheter infection, vertigo, prothrombin time, pulmonary, rectal bleeding, dysuria, nail changes, chest pain, rectal pain, syncope, hypertension, hypoxia, unknown infection, bone pain, pigmentation changes, and urticaria.
Table 10: Laboratory-Related Adverse Reactions Occurring in ≥5% of Patients in the Previously Untreated Advanced Colorectal Cancer Trial
| ||||||
|
† Alanine transaminase | ||||||
|
Laboratory-Related |
Oxaliplatin and FU/LV N=259 |
Irinotecan and FU/LV N=256 |
Oxaliplatin and | |||
|
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 | |
|
Hematology | ||||||
|
Leukopenia |
85 |
20 |
84 |
23 |
76 |
24 |
|
Neutropenia |
81 |
53 |
77 |
44 |
71 |
36 |
|
Thrombocytopenia |
71 |
5 |
26 |
2 |
44 |
4 |
|
Anemia |
27 |
3 |
28 |
4 |
25 |
3 |
|
Hepatic | ||||||
|
Increased AST* |
17 |
1 |
2 |
1 |
11 |
1 |
|
Increased Alkaline Phosphatase |
16 |
0 |
8 |
0 |
14 |
2 |
|
Hyperbilirubinemia |
6 |
1 |
3 |
1 |
3 |
2 |
|
Increased ALT† |
6 |
1 |
2 |
0 |
5 |
2 |
Previously Treated Advanced Colorectal Cancer
The safety of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a randomized trial in patients with refractory and relapsed colorectal cancer [see Clinical Studies (14.3)]. The adverse reaction profile in this trial was similar to that seen in other trials.
Three patients who received Oxaliplatin in the combination arm experienced fatal adverse reactions: gastrointestinal bleeding and dehydration.
Grade 3 and 4 neutropenia were reported in 27% and 17% of patients, respectively, in the Oxaliplatin with fluorouracil/leucovorin combination arm. Grade 3-4 increased serum creatinine occurred in 1% of patients in the Oxaliplatin with combination fluorouracil/leucovorin arm.
Thirteen percent of patients in the Oxaliplatin with fluorouracil/leucovorin combination arm discontinued treatment; the most frequent reasons were gastrointestinal adverse reactions, hematologic adverse reactions and neuropathies.
Tables 11, 12, and 13 summarize the adverse reactions reported in the previously treated advanced colorectal cancer trial.
Table 11: Adverse Reactions Reported in Patients in the Previously Treated Advanced Colorectal Cancer Trial (greater than or equal to 5% of all patients and with greater than or equal to 1% grade 3-4)
| ||||||
|
Adverse Reaction* |
Oxaliplatin + FU/LV N=150 |
Oxaliplatin N=153 |
FU/LV | |||
|
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 (%) | |
|
Neurology | ||||||
|
Neuropathy |
74 |
7 |
76 |
7 |
17 |
0 |
|
Acute |
56 |
2 |
65 |
5 |
10 |
0 |
|
Persistent |
48 |
6 |
43 |
3 |
9 |
0 |
|
Constitutional Symptoms/Pain | ||||||
|
Fatigue |
68 |
7 |
61 |
9 |
52 |
6 |
|
Back Pain |
19 |
3 |
11 |
0 |
16 |
4 |
|
Pain |
15 |
2 |
14 |
3 |
9 |
3 |
|
Gastrointestinal | ||||||
|
Diarrhea |
67 |
11 |
46 |
4 |
44 |
3 |
|
Nausea |
65 |
11 |
64 |
4 |
59 |
4 |
|
Vomiting |
40 |
9 |
37 |
4 |
27 |
4 |
|
Stomatitis |
37 |
3 |
14 |
0 |
32 |
3 |
|
Abdominal Pain |
33 |
4 |
31 |
7 |
31 |
5 |
|
Anorexia |
29 |
3 |
20 |
2 |
20 |
1 |
|
Gastroesophageal Reflux |
5 |
2 |
1 |
0 |
3 |
0 |
|
Hematology/Infection | ||||||
|
Fever |
29 |
1 |
25 |
1 |
23 |
1 |
|
Febrile Neutropenia |
6 |
6 |
0 |
0 |
1 |
1 |
|
Cardiovascular | ||||||
|
Dyspnea |
20 |
4 |
13 |
7 |
11 |
2 |
|
Coughing |
19 |
1 |
11 |
0 |
9 |
0 |
|
Edema |
15 |
1 |
10 |
1 |
13 |
1 |
|
Thromboembolism |
9 |
8 |
2 |
1 |
4 |
2 |
|
Chest Pain |
8 |
1 |
5 |
1 |
4 |
1 |
|
Dermatology/Skin | ||||||
|
Injection Site Reaction |
10 |
3 |
9 |
0 |
5 |
1 |
|
Hepatic/Metabolic/Laboratory/Renal | ||||||
|
Hypokalemia |
9 |
4 |
3 |
2 |
3 |
1 |
|
Dehydration |
8 |
3 |
5 |
3 |
6 |
4 |
| |||
|
† No complete alopecia was reported. | |||
|
Adverse Reaction* |
Oxaliplatin + |
Oxaliplatin |
5-FU/LV |
|
All Grades (%) |
All Grades (%) |
All Grades (%) | |
|
Gastrointestinal | |||
|
Constipation |
32 |
31 |
23 |
|
Dyspepsia |
14 |
7 |
10 |
|
Taste Perversion |
13 |
5 |
1 |
|
Mucositis |
7 |
2 |
10 |
|
Flatulence |
5 |
3 |
6 |
|
Constitutional Symptoms/Pain/Ocular/Visual | |||
|
Headache |
17 |
13 |
8 |
|
Arthralgia |
10 |
7 |
10 |
|
Epistaxis |
9 |
2 |
1 |
|
Abnormal Lacrimation |
7 |
1 |
6 |
|
Rigors |
7 |
9 |
6 |
|
Allergy/Immunology | |||
|
Rhinitis |
15 |
6 |
4 |
|
Allergic Reaction |
10 |
3 |
1 |
|
Rash |
9 |
5 |
5 |
|
Neurology | |||
|
Dizziness |
13 |
7 |
8 |
|
Insomnia |
9 |
11 |
4 |
|
Dermatology/Skin | |||
|
Hand-Foot Syndrome |
11 |
1 |
13 |
|
Flushing |
10 |
3 |
2 |
|
Alopecia† |
7 |
3 |
3 |
|
Pulmonary | |||
|
Upper Respiratory Tract Infection |
10 |
7 |
4 |
|
Pharyngitis |
9 |
2 |
10 |
|
Cardiovascular | |||
|
Peripheral Edema |
10 |
5 |
11 |
|
Hepatic/Metabolic/Laboratory/Renal | |||
|
Hematuria |
6 |
0 |
4 |
|
Dysuria |
6 |
1 |
1 |
Clinically relevant adverse reactions in greater than or equal to 2% and less than 5% of the patients in the Oxaliplatin and fluorouracil/leucovorin combination arm (listed in decreasing order of frequency) were: anxiety, myalgia, erythematous rash, increased sweating, conjunctivitis, weight decrease, dry mouth, rectal hemorrhage, depression, ataxia, ascites, hemorrhoids, muscle weakness, nervousness, tachycardia, abnormal micturition frequency, dry skin, pruritus, hemoptysis, purpura, vaginal hemorrhage, melena, somnolence, pneumonia, proctitis, involuntary muscle contractions, intestinal obstruction, gingivitis, tenesmus, hot flashes, enlarged abdomen, and urinary incontinence.
Table 13: Laboratory-Related Adverse Reactions Occurring in ≥5% of Patients with Previously Treated Advanced Colorectal Cancer
| ||||||
|
† Aspartate transaminase | ||||||
|
Laboratory-Related Adverse Reaction |
Oxaliplatin and FU/LV N=150 |
Oxaliplatin N=153 |
FU/LV N=142 | |||
|
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 (%) |
All Grades (%) |
Grades 3-4 | |
|
Hematology | ||||||
|
Anemia |
81 |
2 |
64 |
1 |
68 |
2 |
|
Leukopenia |
76 |
19 |
13 |
0 |
34 |
1 |
|
Neutropenia |
73 |
44 |
7 |
0 |
25 |
5 |
|
Thrombocytopenia |
64 |
4 |
30 |
3 |
20 |
0 |
|
Hepatic | ||||||
|
Increased ALT* |
31 |
0 |
36 |
1 |
28 |
3 |
|
Increased AST† |
47 |
0 |
54 |
4 |
39 |
2 |
|
Increased Bilirubin |
13 |
1 |
13 |
5 |
22 |
6 |
Additional Adverse Reactions
The following adverse reactions were observed across clinical trials.
Intravenous site reactions
Injection site reaction, including redness, swelling, and pain, can occur with Oxaliplatin. In some cases, skin necrosis has occurred with extravasation.
PRES
PRES occurred in less than 0.1% of patients.
Pulmonary fibrosis and interstitial lung disease
Pulmonary fibrosis, which may be fatal, occurred in less than 1% of patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Oxaliplatin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
•
General: angioedema, anaphylactic shock
•
Cardiovascular: QT prolongation leading to ventricular arrhythmias, including fatal torsade de pointes; bradyarrhythmia
•
Neurological: loss of deep tendon reflexes, dysarthria, Lhermitte's sign, cranial nerve palsies, fasciculations, convulsion
•
Hearing and vestibular system: deafness
•
Infections: septic shock, including fatal outcomes
•
Infusion-related reactions and hypersensitivity reactions: laryngospasm
•
Hepatic and gastrointestinal: severe diarrhea/vomiting resulting in hypokalemia, colitis (including Clostridium difficile diarrhea), metabolic acidosis, ileus, intestinal obstruction, pancreatitis, sinusoidal obstruction syndrome, perisinusoidal fibrosis which rarely may progress, esophagitis
•
Musculoskeletal and connective tissue: rhabdomyolysis, including fatal outcomes
•
Platelet, bleeding, and clotting disorders: immuno-allergic thrombocytopenia, prolonged prothrombin time and INR in patients receiving anticoagulants
•
Blood disorders: secondary leukemia
•
Red blood cell: hemolytic uremic syndrome, immuno-allergic hemolytic anemia
•
Renal: acute tubular necrosis, acute interstitial nephritis, acute renal failure
•
Respiratory: interstitial lung diseases (sometimes fatal) and pneumonia (including fatal outcomes)
•
Vision: decrease of visual acuity, visual field disturbance, optic neuritis and transient vision loss (reversible following treatment discontinuation)
•
Injury, poisoning, and procedural complications: fall-related injuries
Most common adverse reactions (incidence greater than or equal to 40%) were peripheral sensory neuropathy, neutropenia, thrombocytopenia, anemia, nausea, increase in transaminases and alkaline phosphatase, diarrhea, emesis, fatigue, and stomatitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC, Vigilance & Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs that Prolong the QT Interval
QT interval prolongation and ventricular arrhythmias can occur with Oxaliplatin [see Warnings and Precautions (5.7)]. Avoid coadministration of Oxaliplatin with medicinal products with a known potential to prolong the QT interval.
7.2 Use with Nephrotoxic Drugs
Because platinum-containing species are eliminated primarily through the kidney, clearance of these products may be decreased by coadministration of potentially nephrotoxic compounds [see Clinical Pharmacology (12.3)]. Avoid coadministration of Oxaliplatin with medicinal products known to cause nephrotoxicity.
7.3 Use with Anticoagulants
Prolonged prothrombin time and INR occasionally associated with hemorrhage have been reported in patients who received Oxaliplatin with fluorouracil/leucovorin while on anticoagulants [see Warnings and Precautions (5.10), Adverse Reactions (6.2)]. Increase frequency of monitoring in patients who are receiving Oxaliplatin with fluorouracil/leucovorin and oral anticoagulants.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its direct interaction with DNA, Oxaliplatin can cause fetal harm when administered to a pregnant woman. The available human data do not establish the presence or absence of major birth defects or miscarriage related to the use of Oxaliplatin. Reproductive toxicity studies demonstrated adverse effects on embryo-fetal development in rats at maternal doses that were below the recommended human dose based on body surface area (see Data). Advise a pregnant woman of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal data
Pregnant rats were administered oxaliplatin at less than one-tenth the recommended human dose based on body surface area during gestation days (GD)1-5 (preimplantation), GD 6-10, or GD 11-16 (during organogenesis). Oxaliplatin caused developmental mortality (increased early resorptions) when administered on days GD 6-10 and GD 11-16 and adversely affected fetal growth (decreased fetal weight, delayed ossification) when administered on days GD 6-10.
8.2 Lactation
Risk Summary
There are no data on the presence of oxaliplatin or its metabolites in human or animal milk or its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment with Oxaliplatin and for 3 months after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating Oxaliplatin [see Use in Specific Populations (8.1)].
Contraception
Oxaliplatin can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise female patients of reproductive potential to use effective contraception while receiving Oxaliplatin and for 9 months after the final dose.
Males
Based on its mechanism action as a genotoxic drug, advise males with female partners of reproductive potential to use effective contraception while receiving Oxaliplatin and for 6 months after the final dose [see Nonclinical Toxicology (13.1)].
Infertility
Based on animal studies, Oxaliplatin may impair fertility in males and females [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of Oxaliplatin in pediatrics have not been established. Safety and effectiveness were assessed across 4 open-label studies in 235 patients aged 7 months to 22 years with solid tumors.
In a multicenter, open-label, non-comparative, non-randomized study (ARD5531), oxaliplatin was administered to 43 patients with refractory or relapsed malignant solid tumors, mainly neuroblastoma and osteosarcoma. The dose limiting toxicity (DLT) was sensory neuropathy at a dose of 110 mg/m2. The main adverse reactions were: paresthesia (60%, grade 3-4: 7%), fever (40%, grade 3-4: 7%), and thrombocytopenia (40%, grade 3-4: 27%). No responses were observed.
In an open-label non-randomized study (DFI7434), oxaliplatin was administered to 26 pediatric patients with metastatic or unresectable solid tumors, mainly neuroblastoma and ganglioneuroblastoma. The DLT was sensory neuropathy at a dose of 160 mg/m2. No responses were observed.
In an open-label, single-agent study (ARD5021), oxaliplatin was administered to 43 pediatric patients with recurrent or refractory embryonal CNS tumors. The most common adverse reactions reported were: leukopenia (67%, grade 3-4: 12%), anemia (65%, grade 3-4: 5%), thrombocytopenia (65%, grade 3-4: 26%), vomiting (65%, grade 3-4: 7%), neutropenia (58%, grade 3-4: 16%), and sensory neuropathy (40%, grade 3-4: 5%).
In an open-label single-agent study (ARD5530), oxaliplatin was administered to 123 pediatric patients with recurrent solid tumors, including neuroblastoma, osteosarcoma, Ewing sarcoma or peripheral PNET, ependymoma, rhabdomyosarcoma, hepatoblastoma, high grade astrocytoma, Brain stem glioma, low grade astrocytoma, malignant germ cell tumor and other tumors. The most common adverse reactions reported were: sensory neuropathy (52%, grade 3-4: 12%), thrombocytopenia (37%, grade 3-4: 17%), anemia (37%, grade 3-4: 9%), vomiting (26%, grade 3-4: 4%), increased ALT (24%, grade 3-4: 6%), increased AST (24%, grade 3-4: 2%), and nausea (23%, grade 3-4: 3%).
The pharmacokinetic parameters of ultrafiltrable platinum were evaluated in 105 pediatric patients during the first cycle. The mean clearance in pediatric patients estimated by the population pharmacokinetic analysis was 4.7 L/h (%CV, 41%). Mean platinum pharmacokinetic parameters in ultrafiltrate were Cmax of 0.75 ± 0.24 mcg/mL, AUC0-48h of 7.52 ± 5.07 mcg∙h/mL and AUCinf of 8.83 ± 1.57 mcg∙h/mL at 85 mg/m2 of oxaliplatin and Cmax of 1.10 ± 0.43 mcg/mL, AUC0-48h of 9.74 ± 2.52 mcg∙h/mL and AUCinf of 17.3 ± 5.34 mcg∙h/mL at 130 mg/m2 of oxaliplatin.
8.5 Geriatric Use
In the adjuvant treatment trial [see Clinical Studies (14.1)], 400 patients who received Oxaliplatin with fluorouracil/leucovorin were greater than or equal to 65 years. The effect of Oxaliplatin in patients greater than or equal to 65 years was not conclusive. Patients greater than or equal to 65 years receiving Oxaliplatin experienced more diarrhea and grade 3-4 neutropenia (45% vs 39%) compared to patients less than 65 years.
In the previously untreated advanced colorectal cancer trial [see Clinical Studies (14.2)], 99 patients who received Oxaliplatin with fluorouracil and leucovorin were greater than or equal to 65 years. The same efficacy improvements in response rate, time to tumor progression, and overall survival were observed in the greater than or equal to 65 years patients as in the overall study population. Adverse reactions were similar in patients less than 65 and greater than or equal to 65 years, but older patients may have been more susceptible to diarrhea, dehydration, hypokalemia, leukopenia, fatigue, and syncope.
In the previously treated advanced colorectal cancer trial [see Clinical Studies (14.3)], 55 patients who received Oxaliplatin with fluorouracil and leucovorin were greater than or equal to 65 years. No overall differences in effectiveness were observed between these patients and younger adults. Adverse reactions were similar in patients less than 65 and greater than or equal to 65 years, but older patients may have been more susceptible to diarrhea, dehydration, hypokalemia, and fatigue.
No significant effect of age on the clearance of ultrafiltrable platinum has been observed [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
The AUC of unbound platinum in plasma ultrafiltrate was increased in patients with renal impairment [see Clinical Pharmacology (12.3)]. No dose reduction is recommended for patients with mild (creatinine clearance 50 to 79 mL/min) or moderate (creatinine clearance 30 to 49 mL/min) renal impairment, calculated by Cockcroft-Gault equation. Reduce the dose of Oxaliplatin in patients with severe renal impairment (creatinine clearance less than 30 mL/min) [see Dosage and Administration (2.3)].
•
Lactation: Advise not to breastfeed.
REFERENCES SECTION
15 REFERENCES
“OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer Oxaliplatin Injection in combination with fluorouracil and leucovorin every 2 weeks.
•
For adjuvant treatment, continue treatment for up to 12 cycles or unacceptable toxicity.
•
For advanced colorectal cancer, continue treatment until disease progression or unacceptable toxicity.
Day 1
Administer Oxaliplatin 85 mg/m2 as an intravenous infusion over 120 minutes and leucovorin 200 mg/m2 as an intravenous infusion over 120 minutes at the same time in separate bags, followed by fluorouracil 400 mg/m2 as intravenous bolus over 2 to 4 minutes, followed by fluorouracil 600 mg/m2 as a 22-hour continuous infusion.
Day 2
Administer leucovorin 200 mg/m2 as an intravenous infusion over 120 minutes, followed by fluorouracil 400 mg/m2 as intravenous bolus over 2 to 4 minutes, followed by fluorouracil 600 mg/m2 as a 22-hour continuous infusion.
Refer to the prescribing information for fluorouracil and leucovorin for additional information.
2.2 Dosage Modification for Adverse Reactions
Prolongation of infusion time for Oxaliplatin from 2 hours to 6 hours may mitigate acute toxicities, such as non-life threatening infusion-related reactions.
Permanently discontinue Oxaliplatin for any of the following:
•
Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
•
Posterior reversible encephalopathy syndrome (PRES) [see Warnings and Precautions (5.4)]
•
Confirmed interstitial lung disease or pulmonary fibrosis [see Warnings and Precautions (5.5)]
•
Rhabdomyolysis [see Warnings and Precautions (5.8)]
Refer to the fluorouracil and leucovorin prescribing information for dosage modifications for adverse reactions.
Dosage Modifications for Adjuvant Treatment
Dosage modifications for adverse reactions for adjuvant treatment are presented in Table 1.
Table 1: Dosage Modifications for Adjuvant Treatment in Patients with Stage III Colon Cancer|
Adverse Reactions |
Severity |
Oxaliplatin Injection Dosage Modifications |
|
Peripheral Sensory Neuropathy [see Warnings and Precautions (5.2)] |
Persistent Grade 2 |
Consider reducing Oxaliplatin Injection dose to 75 mg/m2. |
|
Persistent Grade 3 |
Consider discontinuing Oxaliplatin Injection. | |
|
Grade 4 |
Discontinue Oxaliplatin Injection. | |
|
Myelosuppression |
Grade 4 neutropenia or febrile neutropenia |
Delay the next dose until neutrophils greater than or equal to 1.5 × 109/L and
platelets greater than or equal to |
|
Grade 3 to 4 thrombocytopenia | ||
|
Gastrointestinal Adverse Reactions |
Grade 3-4 |
After recovery, reduce Oxaliplatin Injection dose to 75 mg/m2 along with a dose reduction of fluorouracil to 300 mg/m2 as an intravenous bolus and 500 mg/m2 as a 22-hour continuous infusion. |
Dosage Modifications for Advanced Colorectal Cancer
Dosage modifications for adverse reactions for advanced colorectal cancer are presented in Table 2.
Table 2: Dosage Modifications for Advanced Colorectal Cancer|
Adverse Reactions |
Severity |
Oxaliplatin Dosage Modifications |
|
Neuropathy |
Persistent Grade 2 |
Consider reducing Oxaliplatin Injection dose to 65 mg/m2. |
|
Persistent Grade 3 |
Consider discontinuing Oxaliplatin Injection. | |
|
Grade 4 |
Discontinue Oxaliplatin Injection | |
|
Myelosuppression |
Grade 4 neutropenia or febrile neutropenia |
Delay the next dose until neutrophils greater than or equal to 1.5 × 109/L and platelets greater than or equal to 75 × 109/L. Reduce Oxaliplatin Injection dose to 65 mg/m2. |
|
[see Warnings and Precautions (5.3), Adverse Reactions (6.1)] |
Grade 3-4 thrombocytopenia | |
|
Gastrointestinal Adverse Reactions |
Grade 3-4 |
After recovery, reduce Oxaliplatin Injection dose to 65 mg/ m2 along with a dose reduction of fluorouracil to 300 mg/m2 as an intravenous bolus and 500 mg/m2 as a 22-hour continuous infusion. |
2.3 Dosage Modifications for Patients with Renal Impairment
In patients with severe renal impairment (creatinine clearance [CLcr] less than 30 mL/min, calculated by the Cockcroft-Gault equation), reduce the Oxaliplatin Injection dose to 65 mg/m2 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.4 Preparation and Administration
•
Oxaliplatin Injection is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
•
Do not freeze.
•
Protect the concentrated solution from light.
•
Dilute concentrated solution with 250 to 500 mL of 5% Dextrose Injection, USP.**Do not dilute with sodium chloride solution or other chloride-containing solutions.**
•
Store diluted solution for no more than 6 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours under refrigeration (2°C to 8°C [36°F to 46°F]). Protection from light is not required.
•
Visually inspect for particulate matter and discoloration prior to administration and discard if present.
•
Do not mix Oxaliplatin or administer Oxaliplatin through the same infusion line concurrently with alkaline medications or media (such as basic solutions of fluorouracil).
•
Flush the infusion line with 5% Dextrose Injection, USP prior to administration of any concomitant medication.
•
Do not use needles or intravenous administration sets containing aluminum parts for the preparation or mixing of Oxaliplatin. Aluminum has been reported to cause degradation of platinum compounds.
•
Administer Oxaliplatin as an intravenous infusion over 120 minutes concurrently with leucovorin over 120 minutes in separate bags.
•
Administer Oxaliplatin Injection 85 mg/m2 as an intravenous infusion over 120 minutes concurrently with leucovorin over 120 minutes in separate bags, followed by fluorouracil on Day 1 of each 14-day cycle. Administer fluorouracil and leucovorin on Day 2 as recommended. (2.1)
•
Adjuvant Treatment: Continue treatment for up to 12 cycles or unacceptable toxicity. (2.1)
•
Advanced Colorectal Cancer: Continue treatment until disease progression or unacceptable toxicity. (2.1)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: 50 mg (5 mg/mL) or 100 mg (5 mg/mL) clear, colorless solution in a single-dose vial.
Injection: 50 mg (5 mg/mL) or 100 mg (5 mg/mL) in a single-dose vial (3)
OVERDOSAGE SECTION
10 OVERDOSAGE
The maximum dose of oxaliplatin that has been administered in a single infusion is 825 mg. Several cases of overdoses have been reported with Oxaliplatin. Adverse reactions observed following an overdosage were grade 4 thrombocytopenia (less than 25,000/mm3) without bleeding, anemia, sensory neuropathy (including paresthesia, dysesthesia, laryngospasm and facial muscle spasms), gastrointestinal disorders (including nausea, vomiting, stomatitis, flatulence, abdomen enlarged and grade 4 intestinal obstruction), grade 4 dehydration, dyspnea, wheezing, chest pain, respiratory failure, severe bradycardia and death.
Closely monitor patients suspected of receiving an overdose, including for the adverse reactions described above and administer appropriate supportive treatment.
DESCRIPTION SECTION
11 DESCRIPTION
Oxaliplatin is a platinum-based drug with the molecular formula C8H14N2O4Pt and the chemical name of cis-[(1 R,2 R)-1,2-cyclohexanediamine-N,N'] [oxalato(2-)-O,O'] platinum. Oxaliplatin is an organoplatinum complex in which the platinum atom is complexed with 1,2-diaminocyclohexane (DACH) and with an oxalate ligand as a leaving group.
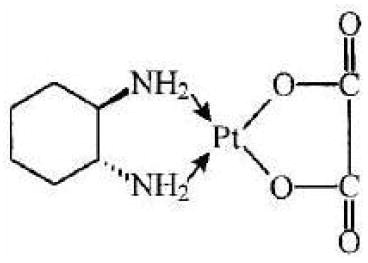
The molecular weight is 397.3. Oxaliplatin is slightly soluble in water at 6 mg/mL, very slightly soluble in methanol, and practically insoluble in ethanol and acetone.
Oxaliplatin Injection, USP, for intravenous use is supplied in vials containing 50 mg or 100 mg of oxaliplatin as a sterile, preservative-free, aqueous solution at a concentration of 5 mg/mL. Water for Injection, USP is present as an inactive ingredient.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oxaliplatin undergoes nonenzymatic conversion in physiologic solutions to active derivatives via displacement of the labile oxalate ligand. Several transient reactive species are formed, including monoaquo and diaquo DACH platinum, which covalently bind with macromolecules. Both inter- and intrastrand Pt-DNA crosslinks are formed. Crosslinks are formed between the N7 positions of two adjacent guanines (GG), adjacent adenine-guanines (AG), and guanines separated by an intervening nucleotide (GNG). These crosslinks inhibit DNA replication and transcription. Cytotoxicity is cell-cycle nonspecific.
In vivo studies have shown antitumor activity of oxaliplatin against colon carcinoma. In combination with fluorouracil, oxaliplatin exhibits in vitro and in vivo antiproliferative activity greater than either compound alone in several tumor models (HT29 [colon], GR [mammary], and L1210 [leukemia]).
12.2 Pharmacodynamics
A pharmacodynamic relationship between platinum ultrafiltrate levels and clinical safety and effectiveness has not been established.
12.3 Pharmacokinetics
The reactive oxaliplatin derivatives are present as a fraction of the unbound platinum in plasma ultrafiltrate. After a single 2-hour intravenous infusion of Oxaliplatin at a dose of 85 mg/m2, pharmacokinetic parameters expressed as ultrafiltrable platinum were Cmax of 0.814 mcg/mL and volume of distribution of 440 L.
Interpatient and intrapatient variability in ultrafiltrable platinum exposure (AUC0-48hr) assessed over 3 cycles was 23% and 6%, respectively.
Distribution
At the end of a 2-hour infusion of Oxaliplatin, approximately 15% of the administered platinum is present in the systemic circulation. The remaining 85% is rapidly distributed into tissues or eliminated in the urine. The decline of ultrafiltrable platinum levels following Oxaliplatin administration is triphasic, including two distribution phases (t1/2α; 0.43 hours and t1/2β; 16.8 hours).
In patients, plasma protein binding of platinum is irreversible and is greater than 90%. The main binding proteins are albumin and gamma-globulins.
Platinum also binds irreversibly and accumulates (approximately 2-fold) in erythrocytes, where it appears to have no relevant activity. No platinum accumulation was observed in plasma ultrafiltrate following 85 mg/m2 every two weeks.
Elimination
The decline of ultrafiltrable platinum concentrations from plasma is characterized by a long terminal elimination phase (t1/2γ; 392 hours).
Metabolism
Oxaliplatin undergoes rapid and extensive nonenzymatic biotransformation. There is no evidence of cytochrome P450-mediated metabolism in vitro.
Up to 17 platinum-containing derivatives have been observed in plasma ultrafiltrate samples from patients, including several cytotoxic species (monochloro DACH platinum, dichloro DACH platinum, and monoaquo and diaquo DACH platinum) and a number of noncytotoxic, conjugated species.
Excretion
The major route of platinum elimination is renal excretion. At five days after a single 2-hour infusion of Oxaliplatin, urinary elimination accounted for about 54% of the platinum eliminated, with fecal excretion accounting for only about 2%. Platinum was cleared from plasma at a rate (10 to 17 L/h) that was similar to or exceeded the average human glomerular filtration rate (GFR; 7.5 L/h). The renal clearance of ultrafiltrable platinum is significantly correlated with GFR.
Special Populations
Sex
There was no significant effect of sex on the clearance of ultrafiltrable platinum.
Patients with renal impairment
Patients with normal function (CLcr greater than 80 mL/min) and patients with mild (CLcr=50- 80 mL/min) and moderate (CLcr equal to 30-49 mL/min) renal impairment received Oxaliplatin 85 mg/m2 and those with severe (CLcr less than 30 mL/min) renal impairment received Oxaliplatin 65 mg/m2. Mean dose adjusted AUC of unbound platinum was 40%, 95%, and 342% higher for patients with mild, moderate, and severe renal impairment, respectively, compared to patients with normal renal function. Mean dose adjusted Cmax of unbound platinum appeared to be similar among the normal, mild and moderate renal function groups, but was 38% higher in the severe group than in the normal group [see Dosage and Administration (2.3)].
Drug Interaction Studies
No pharmacokinetic interaction between Oxaliplatin 85 mg/m2 and infusional fluorouracil has been observed in patients treated every 2 weeks, but increases of fluorouracil plasma concentrations by approximately 20% have been observed with doses of 130 mg/m2 of Oxaliplatin administered every 3 weeks.
In vitro platinum was not displaced from plasma proteins by the following medications: erythromycin, salicylate, sodium valproate, granisetron, and paclitaxel.
In vitro Oxaliplatin does not inhibit human cytochrome P450 isoenzymes.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adjuvant Treatment with Oxaliplatin in Combination with Fluorouracil
and Leucovorin
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in an international, multicenter, randomized (1:1) trial (The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer [MOSAIC], NCT00275210) in patients with stage II (Dukes' B2) or III (Dukes' C) colon cancer who had undergone complete resection of the primary tumor. Patients were randomized to receive Oxaliplatin with fluorouracil/leucovorin or fluorouracil/leucovorin alone for a total of 6 months (i.e., 12 cycles). Table 14 shows the dosing regimens for the two arms.
Eligible patients were between 18 and 75 years of age, had histologically proven stage II (T3-T4 N0 M0; Dukes' B2) or III (any T N1-2 M0; Dukes' C) colon carcinoma (with the inferior pole of the tumor above the peritoneal reflection, i.e., greater than or equal to 15 cm from the anal margin) and had undergone (within 7 weeks prior to randomization) complete resection of the primary tumor without gross or microscopic evidence of residual disease and carcino-embyrogenic antigen (CEA) less than 10 ng/mL. Additional eligibility criteria were no prior chemotherapy, immunotherapy or radiotherapy; Eastern Cooperative Oncology Group performance status of 0, 1, or 2 (Karnofsky Performance Status greater than or equal to 60%); no pre-existing neuropathy; and absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, serum creatinine less than or equal to 1.25 × upper limit normal (ULN), total bilirubin less than 2 × ULN, and aspartate transaminase (AST)/alanine transaminase (ALT) less than 2 × ULN. The major efficacy outcome was 3-year disease-free survival (DFS).
Table 14: Dosing Regimens in Adjuvant Treatment Study|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + FU/LV (FOLFOX4) (N=1123) |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV: 200 mg/m2 |
every 2 weeks |
|
FU/LV (N=1123) |
Day 1: LV: 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600
mg/m2 (22-hour infusion) |
every 2 weeks |
There were 2,246 patients enrolled, of whom 1,347 (60%) had Stage III disease. Tables 15 and 16 show the baseline characteristics and exposure to Oxaliplatin.
Table 15: Baseline Characteristics in Adjuvant Treatment Study|
Oxaliplatin + Infusional FU/LV |
Infusional FU/LV | |
|
Sex: Male (%) |
56.1 |
52.4 |
|
Female (%) |
43.9 |
47.6 |
|
Median age (years) |
61.0 |
60.0 |
|
<65 years of age (%) |
64.4 |
66.2 |
|
≥65 years of age (%) |
35.6 |
33.8 |
|
KPS (%) | ||
|
100 |
29.7 |
30.5 |
|
90 |
52.2 |
53.9 |
|
80 |
4.4 |
3.3 |
|
70 |
13.2 |
11.9 |
|
≤60 |
0.6 |
0.4 |
|
Primary site (%) | ||
|
Colon including cecum |
54.6 |
54.4 |
|
Sigmoid |
31.9 |
33.8 |
|
Recto sigmoid |
12.9 |
10.9 |
|
Other including rectum |
0.6 |
0.9 |
|
Bowel obstruction (%) | ||
|
Yes |
17.9 |
19.3 |
|
Perforation (%) | ||
|
Yes |
6.9 |
6.9 |
|
Stage at Randomization (%) | ||
|
II (T=3,4 N=0, M=0) |
40.1 |
39.9 |
|
III (T=any, N=1,2, M=0) |
59.6 |
59.3 |
|
IV (T=any, N=any, M=1) |
0.4 |
0.8 |
|
Staging – T (%) | ||
|
T1 |
0.5 |
0.7 |
|
T2 |
4.5 |
4.8 |
|
T3 |
76.0 |
75.9 |
|
T4 |
19.0 |
18.5 |
|
Staging – N (%) | ||
|
N0 |
40.2 |
39.9 |
|
N1 |
39.4 |
39.4 |
|
N2 |
20.4 |
20.7 |
|
Staging – M (%) | ||
|
M1 |
0.4 |
0.8 |
|
Oxaliplatin + |
Infusional | |
|
Median Relative Dose Intensity (%) | ||
|
FU |
84.4 |
97.7 |
|
Oxaliplatin |
80.5 |
N/A |
|
Median Number of Cycles |
12 |
12 |
|
Median Number of Cycles with Oxaliplatin |
11 |
N/A |
The median duration of follow-up was approximately 77 months. In the overall and the stage III colon cancer populations, DFS was statistically significantly improved in the Oxaliplatin - containing arm compared to fluorouracil/leucovorin alone; however, a statistically significant improvement in DFS was not observed in Stage II patients. No significant differences in overall survival (OS) were detected in the overall population or those with Stage III disease. Table 17 and Figures 1 and 2 summarize the 5-year DFS rates in the overall randomized population and in patients with stage II and III disease based on an intention-to-treat (ITT) analysis.
Table 17: Summary of DFS Analysis in Adjuvant Treatment Study – ITT Population|
A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
|
Data cut off for disease-free survival June 1, 2006 | ||
|
Parameter |
Oxaliplatin + |
Infusional |
|
Overall | ||
|
Number of patients |
1123 |
1123 |
|
Number of events – relapse or death (%) |
304 (27.1) |
360 (32.1) |
|
5-yr Disease-free survival % (95% CI) |
73.3 (70.7, 76.0) |
67.4 (64.6, |
|
Hazard ratio (95% CI) |
0.80 (0.68, 0.93) | |
|
Stratified Log rank test |
p=0.003 | |
|
Stage III (Dukes' C) | ||
|
Number of patients |
672 |
675 |
|
Number of events – relapse or death (%) |
226 (33.6) |
271 (40.1) |
|
5-yr Disease-free survival % (95% CI) |
66.4 (62.7, 70.0) |
58.9 (55.2, 62.7) |
|
Hazard ratio (95% CI) |
0.78 (0.65, 0.93) | |
|
Log rank test |
p=0.005 | |
|
Stage II (Dukes' B2) | ||
|
Number of patients |
451 |
448 |
|
Number of events – relapse or death (%) |
78 (17.3) |
89 (19.9) |
|
5-yr Disease-free survival % (95% CI) |
83.7 (80.2, 87.1) |
79.9 (76.2, 83.7) |
|
Hazard ratio (95% CI) |
0.84 (0.62, 1.14) | |
|
Log rank test |
p=0.258 |
Figure 1: Kaplan-Meier Curves of Disease-Free Survival (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
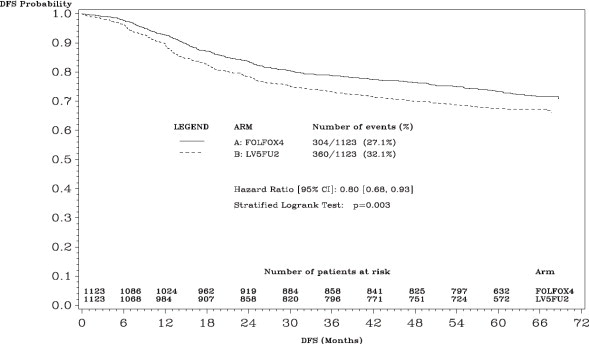
Figure 2: Kaplan-Meier Curves of Disease-Free Survival in Stage III Patients (cutoff: 1 June 2006) in Adjuvant Treatment Trial - ITT Population
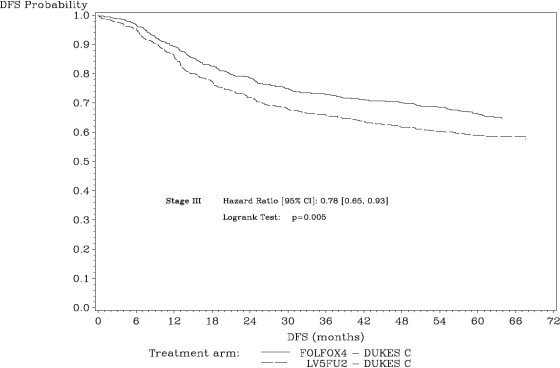
Table 18 summarizes the OS results in the overall randomized population and in patients with stage II and III disease, based on the ITT analysis.
Table 18: Summary of OS Analysis in Adjuvant Treatment - ITT Population|
A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
|
Data cut off for overall survival January 16, 2007 | ||
|
Parameter |
Oxaliplatin + |
Infusional FU/LV |
|
Overall | ||
|
Number of patients |
1123 |
1123 |
|
Number of death events (%) |
245 (21.8) |
283 (25.2) |
|
Hazard ratio (95% CI) |
0.84 (0.71, 1.00) | |
|
Stage III (Dukes' C) | ||
|
Number of patients |
672 |
675 |
|
Number of death events (%) |
182 (27.1) |
220 (32.6) |
|
Hazard ratio (95% CI) |
0.80 (0.65, 0.97) | |
|
Stage II (Dukes' B2) | ||
|
Number of patients |
451 |
448 |
|
Number of death events (%) |
63 (14.0) |
63 (14.1) |
|
Hazard ratio (95% CI) |
1.00 (0.70, 1.41) |
14.2 Previously Untreated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a North American, multicenter, open-label, randomized, active-controlled trial (A Randomized Phase III Trial of Three Different Regimens of CPT-11 Plus 5-Fluorouracil and Leucovorin Compared to 5-Fluorouracil and Leucovorin in Patients with Advanced Adenocarcinoma of the Colon and Rectum; NCT00003594). The trial included 7 arms at different times during its conduct, four of which were closed due to either changes in the standard of care, toxicity, or simplification. During the trial, the control arm was changed to irinotecan with fluorouracil/leucovorin.
The results reported below compared the efficacy of Oxaliplatin with fluorouracil/leucovorin and Oxaliplatin with irinotecan to an approved control regimen of irinotecan with fluorouracil/leucovorin in 795 concurrently randomized patients previously untreated for locally advanced or metastatic colorectal cancer. Table 19 presents the dosing regimens for the three arms. After completion of enrollment, the dose of irinotecan with fluorouracil/leucovorin was decreased due to toxicity.
Eligible patients were at least 18 years of age; had known locally advanced, locally recurrent, or metastatic colorectal adenocarcinoma not curable by surgery or amenable to radiation therapy; with an Eastern Cooperative Oncology Group (ECOG) performance status ≤0, 1, or 2. Patients had to have absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, hemoglobin greater than or equal to 9.0 g/dL, creatinine less than or equal to 1.5 × upper limit of normal (ULN), total bilirubin less than or equal to 1.5 mg/dL, aspartate transaminase (AST) less than or equal to 5 × ULN, and alkaline phosphatase less than or equal to 5 × ULN. Patients may have received adjuvant treatment for resected Stage II or III disease without recurrence within 12 months. Randomization was stratified by ECOG performance status (0, 1 vs 2), prior adjuvant chemotherapy (yes vs no), prior immunotherapy (yes vs no), and age (less than 65 vs greater than or equal to 65 years). Although no post study treatment was specified in the protocol, 65% to 72% of patients received additional post study chemotherapy after study treatment discontinuation on all arms. Fifty-eight percent of patients on the Oxaliplatin with fluorouracil/leucovorin arm received an irinotecan-containing regimen and 23% of patients on the irinotecan with fluorouracil/leucovorin arm received an oxaliplatin-containing regimen. The main efficacy outcome measure was 3-year disease-free survival (DFS) and additional efficacy outcome measures were overall survival (OS).
Table 19: Dosing Regimens for Previously Untreated Advanced Colorectal Cancer Clinical Trial|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + FU/LV |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) Day 2: LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) |
every 2 weeks |
|
Irinotecan + |
Day 1: irinotecan 125 mg/m2 as a 90-min infusion + LV 20 mg/m2 as a 15-min infusion or intravenous push, followed by FU 500 mg/m2 intravenous bolus weekly x 4 |
every 6 weeks |
|
Oxaliplatin+ |
Day 1: Oxaliplatin: 85 mg/m2 intravenous(2-hour infusion) + irinotecan 200 mg/m2 intravenous over 30 minutes |
every 3 weeks |
Table 20 presents the baseline characteristics.
Table 20: Baseline Characteristics for Previously Untreated Advanced Colorectal Cancer Clinical Trial|
Oxaliplatin + FU/LV N=267 |
Irinotecan + FU/LV N=264 |
Oxaliplatin + Irinotecan | |
|
Sex: Male (%) |
58.8 |
65.2 |
61.0 |
|
Female (%) |
41.2 |
34.8 |
39.0 |
|
Median age (years) |
61.0 |
61.0 |
61.0 |
|
<65 years of age (%) |
61 |
62 |
63 |
|
≥65 years of age (%) |
39 |
38 |
37 |
|
ECOG (%) | |||
|
0-1 |
94.4 |
95.5 |
94.7 |
|
2 |
5.6 |
4.5 |
5.3 |
|
Involved organs (%) | |||
|
Colon only |
0.7 |
0.8 |
0.4 |
|
Liver only |
39.3 |
44.3 |
39.0 |
|
Liver + other |
41.2 |
38.6 |
40.9 |
|
Lung only |
6.4 |
3.8 |
5.3 |
|
Other (including lymph nodes) |
11.6 |
11.0 |
12.9 |
|
Not reported |
0.7 |
1.5 |
1.5 |
|
Prior radiation (%) |
3.0 |
1.5 |
3.0 |
|
Prior surgery (%) |
74.5 |
79.2 |
81.8 |
|
Prior adjuvant (%) |
15.7 |
14.8 |
15.2 |
The median number of cycles administered per patient was 10 (23.9 weeks) for the Oxaliplatin plus fluorouracil/leucovorin regimen, 4 (23.6 weeks) for the irinotecan plus fluorouracil/leucovorin regimen, and 7 (21.0 weeks) for the Oxaliplatin plus irinotecan regimen.
Patients who received Oxaliplatin with fluorouracil/leucovorin had a significantly longer time to tumor progression based on investigator assessment, longer OS, and a significantly higher confirmed response rate based on investigator assessment compared to patients who received irinotecan with fluorouracil/leucovorin. Efficacy results are summarized in Table 21 and Figure 3.
Table 21: Efficacy Results for Previously Untreated Advanced Colorectal Cancer Trial
| |||
|
† A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV. ‡ Based on all patients with measurable disease at baseline. | |||
|
The numbers in the response rate and TTP analysis are based on unblinded investigator assessment. | |||
|
Oxaliplatin + FU/LV N=267 |
Irinotecan + FU/LV N=264 |
Oxaliplatin + | |
|
Survival (ITT) | |||
|
Number of deaths (%) |
155 (58.1) |
192 (72.7) |
175 (66.3) |
|
Median survival (months) |
19.4 |
14.6 |
17.6 |
|
Hazard ratio (95% CI) |
0.65 (0.53, 0.80)* |
- | |
|
P-value |
<0.0001* |
- | |
|
TTP (ITT, investigator assessment) | |||
|
Percentage of progressors |
82.8 |
81.8 |
89.4 |
|
Median TTP (months) |
8.7 |
6.9 |
6.5 |
|
Hazard ratio (95% CI) |
0.74 (0.61, 0.89)* |
- | |
|
P-value |
0.0014* |
- | |
|
Response Rate (investigator assessment)****‡ | |||
|
Patients with measurable disease |
210 |
212 |
215 |
|
Complete response, N (%) |
13 (6.2) |
5 (2.4) |
7 (3.3) |
|
Partial response, N (%) |
82 (39.0) |
64 (30.2) |
67 (31.2) |
|
Complete and partial response, N (%) |
95 (45.2) |
69 (32.5) |
74 (34.4) |
|
95% CI |
(38.5, 52.0) |
(26.2, 38.9) |
(28.1, 40.8) |
|
P-value |
0.0080* |
- |
Figure 3: Kaplan-Meier Curves for Overall Survival in Previously Untreated Advanced Colorectal Cancer Trial
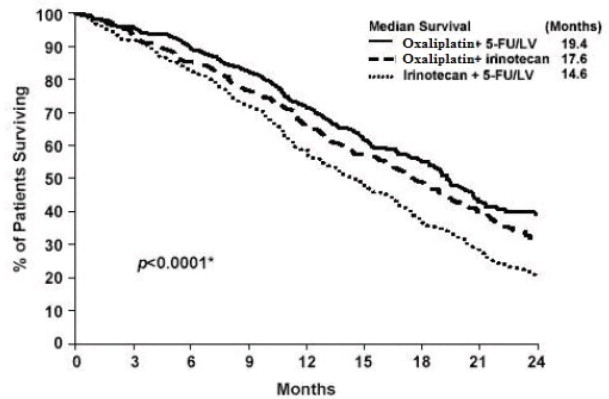
*Log rank test comparing Oxaliplatin plus 5-FU/LV to irinotecan plus 5-FU/LV
In descriptive subgroup analyses, the improvement in overall survival (OS) for Oxaliplatin with fluorouracil/leucovorin compared to irinotecan with fluorouracil/leucovorin appeared to be maintained across age groups, prior adjuvant treatment, number of organs involved and both sexes; however, the effect appeared larger among women than men.
14.3 Previously Treated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a multicenter, open-label, randomized, three-arm controlled trial was conducted in the US and Canada in patients with advanced colorectal cancer who had relapsed/progressed during or within 6 months of first-line treatment with bolus fluorouracil/leucovorin and irinotecan (A multicenter, open-label, randomized, three-arm study of 5-fluorouracil (5-FU)
- leucovorin (LV) or oxaliplatin or a combination of 5-FU/LV + oxaliplatin as second-line treatment of metastatic colorectal carcinoma: NCT00008281). Patients were randomized to one of three regimens; the dosing regimens are presented in Table 22. Eligible patients were at least 18 years of age, had unresectable, measurable, histologically proven colorectal adenocarcinoma, with a Karnofsky performance status (KPS) greater than 50%. Patients had to have aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase less than or equal to 2× upper limit of normal (ULN), unless liver metastases were present and documented at baseline by CT or MRI scan, in which case less than or equal to 5× ULN was permitted. Prior radiotherapy was permitted if it had been completed at least 3 weeks before randomization. The main efficacy outcome measure was 3-year disease-free survival (DFS) and an additional outcome measure was overall survival (OS).
|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) Day 2: LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) |
every 2 weeks |
|
FU/LV |
Day 1: LV 200 mg/m2 (2-hour infusion), followed by Day 2: LV 200 mg/m2 (2-hour infusion), followed by |
every 2 weeks |
|
Oxaliplatin |
Day 1: Oxaliplatin 85 mg/m2 (2-hour infusion) |
every 2 weeks |
Patients must have had at least one unidimensional lesion measuring greater than or equal to 20 mm using conventional CT or MRI scans or greater than or equal to10 mm using a spiral CT scan. Tumor response and progression were assessed every 3 cycles (6 weeks) using the Response Evaluation Criteria in Solid Tumors (RECIST) until radiological documentation of progression or for 13 months following the first dose of study drug(s), whichever came first. Confirmed responses were based on two tumor assessments separated by at least 4 weeks.
Baseline characteristics are shown in Table 23.
Table 23: Baseline Characteristics in Refractory and Relapsed Colorectal Cancer Trial|
Oxaliplatin+ |
Oxaliplatin |
FU/LV | |
|
Sex: Male (%) |
57.2 |
60.9 |
54.3 |
|
Female (%) |
42.8 |
39.1 |
45.7 |
|
Median age (years) |
59.0 |
61.0 |
60.0 |
|
Range |
22-88 |
27-79 |
21-80 |
|
Race (%) | |||
|
Caucasian |
88.8 |
84.6 |
87.4 |
|
Black |
5.9 |
7.1 |
7.9 |
|
Asian |
2.6 |
2.6 |
1.3 |
|
Other |
2.6 |
5.8 |
3.3 |
|
KPS (%) | |||
|
70-100 |
95.4 |
92.3 |
94.7 |
|
50-60 |
2.0 |
4.5 |
2.6 |
|
Not reported |
2.6 |
3.2 |
2.6 |
|
Prior radiotherapy (%) |
25.0 |
19.2 |
25.2 |
|
Prior pelvic radiation (%) |
21.1 |
13.5 |
18.5 |
|
Number of metastatic sites (%) | |||
|
1 |
25.7 |
31.4 |
27.2 |
|
≥ 2 |
74.3 |
67.9 |
72.2 |
|
Liver involvement (%) | |||
|
Liver only |
18.4 |
25.6 |
22.5 |
|
Liver + other |
53.3 |
59.0 |
60.3 |
The median number of cycles administered per patient was 6 for the Oxaliplatin and fluorouracil/leucovorin combination and 3 each for fluorouracil/leucovorin alone and Oxaliplatin alone. Patients treated with the combination of Oxaliplatin and fluorouracil/leucovorin had an increased response rate compared to patients given fluorouracil/leucovorin or oxaliplatin alone. Efficacy results are summarized in Tables 24 and 25.
Table 24: Response Rates in Refractory and Relapsed Colorectal Cancer Clinical Trial - ITT Analysis|
Best Response |
Oxaliplatin + |
Oxaliplatin N=156 |
FU/LV |
|
Complete Response |
0 |
0 |
0 |
|
Partial Response |
13 (9%) |
2 (1%) |
0 |
|
P-value |
0.0002 FU/LV vs Oxaliplatin + FU/LV | ||
|
95% CI |
4.6%, 14.2% |
0.2%, 4.6% |
0, 2.4% |
| |||
|
Arm |
Oxaliplatin + |
Oxaliplatin |
FU/LV |
|
Number of progressors |
50 |
101 |
74 |
|
Number of patients with no radiological evaluation beyond baseline |
17 (11%) |
16 (10%) |
22 (15%) |
|
Median TTP (months) |
4.6 |
1.6 |
2.7 |
|
95% CI |
4.2, 6.1 |
1.4, 2.7 |
1.8, 3.0 |
At the time of the interim analysis 49% of the radiographic progression events had occurred. In this interim analysis an estimated 2-month increase in median time to radiographic progression was observed compared to fluorouracil/leucovorin alone.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients of the potential risk of hypersensitivity and that Oxaliplatin is contraindicated in patients with a history of hypersensitivity reactions to oxaliplatin and other platinum-based drugs. Instruct patients to seek immediate medical attention for signs of severe hypersensitivity reaction such as chest tightness; shortness of breath; wheezing; dizziness or faintness; or swelling of the face, eyelids, or lips [see Warnings and Precautions (5.1)].
Peripheral Sensory Neuropathy
Advise patients of the risk of acute reversible or persistent-type neurosensory toxicity. Advise patients to avoid cold drinks, use of ice, and exposure of skin to cold temperature or cold objects [see Warnings and Precautions (5.2)].
Myelosuppression
Inform patients that Oxaliplatin can cause low blood cell counts and the need for frequent monitoring of blood cell counts. Advise patients to contact their healthcare provider immediately for bleeding, fever, particularly if associated with persistent diarrhea, or symptoms of infection develop [see Warnings and Precautions (5.3)].
Posterior Reversible Encephalopathy Syndrome
Advise patients of the potential effects of vision abnormalities, in particular transient vision loss (reversible following therapy discontinuation), which may affect the patients' ability to drive and use machines [see Warnings and Precautions (5.4)].
Pulmonary Toxicity
Advise patients to report immediately to their healthcare provider any persistent or recurrent respiratory symptoms, such as non-productive cough and dyspnea [see Warnings and Precautions (5.5)].
Hepatotoxicity
Advise patients to report signs or symptoms of hepatic toxicity to their healthcare provider [see Warnings and Precautions (5.6)].
QT Interval Prolongation
Advise patients that Oxaliplatin can cause QTc interval prolongation and to inform their physician if they have any symptoms, such as syncope [see Warnings and Precautions (5.7)].
Rhabdomyolysis
Advise patients to contact their healthcare provider immediately for new or worsening signs or symptoms of muscle toxicity, dark urine, decreased urine output, or the inability to urinate [see Warnings and Precautions (5.8)].
Hemorrhage
Advise patients that Oxaliplatin may increase the risk of bleeding and to promptly inform their healthcare provider of any bleeding episodes [see Warnings and Precautions (5.9)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.10), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with Oxaliplatin and for 9 months after the final dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Oxaliplatin and for 6 months after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with Oxaliplatin and for 3 months after the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential that Oxaliplatin may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Gastrointestinal
Advise patients to contact their healthcare provider for persistent vomiting, diarrhea, or signs of dehydration [see Adverse Reactions (6.1)].
Drug Interactions
Inform patients about the risk of drug interactions and the importance of providing a list of prescription and nonprescription drugs to their healthcare provider [see Drug Interactions (7)].

For Product Inquiry:
1-800-551-7176 or www.fresenius-kabi.com/us
451426C
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
Oxaliplatin Injection (ox-AL-i-PLA-tin)
for intravenous use
What is the most important information I should know about Oxaliplatin Injection?
Oxaliplatin Injection can cause serious allergic reactions, including allergic reactions that can lead to death. Oxaliplatin is a platinum-based medicine. Serious allergic reactions including death can happen in people who take Oxaliplatin and who have had previous allergic reactions to platinum- based medicines. Serious allergic reactions can happen within a few minutes of your Oxaliplatin infusion or any time during your treatment with Oxaliplatin Injection.
Get emergency help right away if you:
•
**have trouble breathing**
•
**feel like your throat is closing up**
Call your doctor right away if you have any of the following signs or symptoms of an allergic reaction:
•
rash
•
flushed face
•
hives
•
itching
•
swelling of your lips or tongue
•
sudden cough
•
dizziness or feel faint
•
sweating
•
chest pain
See**"What are the possible side effects of Oxaliplatin Injection?"** for information about other serious side effects.
What is Oxaliplatin Injection?
Oxaliplatin is an anti-cancer (chemotherapy) medicine that is used with other anti-cancer medicines called fluorouracil and leucovorin to treat people with:
•
stage III colon cancer after surgery to remove the tumor
•
advanced colon or rectal cancer (colorectal cancer)
It is not known if Oxaliplatin Injection is effective in children.
Who should not receive Oxaliplatin Injection?
Do not receive Oxaliplatin Injection if you are allergic to any of the ingredients in Oxaliplatin Injection or other medicines that contain platinum. See the end of this leaflet for a complete list of the ingredients Oxaliplatin Injection.
Ask your doctor if you are not sure if you take a medicine that contains platinum.
What should I tell my doctor before receiving Oxaliplatin Injection?
Before receiving Oxaliplatin Injection, tell your doctor about all of your medical conditions, including if you:
•
have an infection
•
have lung, liver, or kidney problems
•
have bleeding problems
•
have or had heart problems such as an abnormal heart test called an electrocardiogram (ECG or EKG), a condition called long QT syndrome, an irregular or slow heartbeat, or a family history of heart problems.
•
have had changes in the level of certain blood salt (electrolytes) levels, including potassium, magnesium, and calcium
•
are pregnant or plan to become pregnant. Oxaliplatin Injection may harm your unborn baby. Tell your doctor right away if you become pregnant or think you may be pregnant during treatment with Oxaliplatin Injection.
•
if you are able to become pregnant, your doctor may do a pregnancy test before you start treatment with Oxaliplatin Injection and for 9 months after the final dose. Talk to your doctor about forms of birth control that may be right for you.
•
Females who are able to become pregnant should avoid becoming pregnant and should use effective birth control during treatment with Oxaliplatin Injection and for 9 months after the final dose. Talk to your doctor about forms of birth control that may be right for you.
•
Males with female partners who are pregnant or able to become pregnant should use effective birth control during treatment with Oxaliplatin Injection and for 6 months after the final dose.
•
Oxaliplatin Injection may cause fertility problems in males and females. Talk to your doctor if this is a concern for you.
•
are breastfeeding or plan to breastfeed. It is not known if Oxaliplatin passes into your breast milk. Do not breastfeed during treatment with Oxaliplatin Injection and for 3 months after the final dose.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How will I receive Oxaliplatin Injection?
•
Oxaliplatin Injection is given to you into your vein through an intravenous (IV) tube.
•
Your doctor will prescribe Oxaliplatin Injection in a dose that is right for you.
•
Your doctor may change how often you receive Oxaliplatin Injection, your dose, or how long your infusion will take.
•
You and your doctor will decide how many Oxaliplatin Injection treatments you will receive.
•
It is very important that you do exactly what your doctor and nurse tell you to do.
•
Some medicines may be given to you before Oxaliplatin Injection to help prevent nausea and vomiting.
•
Each treatment course is given to you over 2 days. You will receive Oxaliplatin Injection on the first day only.
•
There are usually 14 days between each chemotherapy treatment course.
•
It is important for you to keep all of your medical appointments. Call your doctor if you miss an appointment. There may be special instructions for you.
Treatment Day 1:
o
Oxaliplatin and leucovorin will be given through a thin plastic tube into a vein (intravenous infusion or IV) and given for 2 hours. You will be watched by a healthcare provider during this time.
o
Right after the Oxaliplatin and leucovorin are given, 2 doses of fluorouracil will be given. The first dose is given right away into your IV tube. The second dose will be given into your IV tube over the next 22 hours, using a pump device.
Treatment Day 2:
Youwill not get Oxaliplatin on Day 2. Leucovorin and fluorouracil will be given the same way as on Day 1.
The fluorouracil will be given through your IV with a pump. If you have any problems with the pump or the tube, call your doctor, your nurse, or the person who is responsible for your pump. Do not let anyone other than a healthcare provider touch your infusion pump or tubing.
What should I avoid while receiving Oxaliplatin Injection?
•
Avoid cold temperatures and cold objects. Cover your skin if you go outdoors in cold temperatures.
•
Do not drink cold drinks or use ice cubes in drinks.
•
Do not put ice or ice packs on your body.
•
Oxaliplatin Injection can cause dizziness, vision problems, or vision loss that can affect your ability to drive or use machines. You should not drive or operate machinery if you develop these symptoms while receiving Oxaliplatin Injection.
See**“How can I reduce the side effects caused by cold temperatures?”** for more information.
Talk with your doctor and nurse about your level of activity during treatment with Oxaliplatin Injection. Follow their instructions.
What are the possible side effects of Oxaliplatin Injection?
Oxaliplatin Injection can cause serious side effects, including:
•
**See "****What is the most important information I should know about Oxaliplatin Injection?****"**
•
**Nerve problems.** Oxaliplatin Injection can affect how your nerves work and make you feel. Nerve problems may happen with the first treatment or within two days after your treatment of Oxaliplatin Injection. Nerve problems may last a short time (acute) or may become persistent. Symptoms may improve after stopping treatment with Oxaliplatin Injection. Exposure to cold or cold objects may cause or worsen nerve problems. Tell your doctor right away if you get any signs of nerve problems, including:
o
very sensitive to cold temperatures and cold objects
o
trouble breathing, swallowing, or saying words, jaw tightness, odd feelings in your tongue, or chest pressure
o
pain, tingling, burning (pins and needles, numb feeling) in your hands, feet, or around your mouth or throat, which may cause problems walking, fall, or performing activities of daily living
For information on ways to lessen or help with the nerve problems, see the end of this leaflet,“How can I reduce the side effects caused by cold temperatures?”
•
**Posterior Reversible Encephalopathy Syndrome (PRES)**. PRES is a rare condition that affects the brain. Tell your doctor right away if you have any of the following signs and symptoms of PRES:
o
headache
o
confusion or a change in the way you think
o
seizures
o
vision problems, such as blurriness or vision loss
•
**Low blood cell counts (myelosuppression)**. Oxaliplatin Injection when used with fluorouracil and leucovorin can cause low blood cells counts. Low blood cell counts are common with Oxaliplatin Injection when used with fluorouracil and leucovorin and can lead to serious infection and death. Tell your doctor right away if you have a fever greater than 100.9°F (38.3°C) or a prolonged fever greater than 100.4°F (38°C) for more than one hour (febrile neutropenia). Call your doctor right away if you get any of the following signs of infection:
o
chills or shivering
o
pain on swallowing
o
sore throat
o
cough that brings up mucus
o
burning or pain on urination
o
redness or swelling at intravenous site
o
persistent diarrhea
•
**Risk of new cancers.** Leukemia, a form of blood cancer, has been reported in patients after taking Oxaliplatin Injection in combination with certain other medicines. Talk to your doctor about the potential for increased risk of this type of cancer when taking Oxaliplatin Injection and certain other medicines.
•
**Lung problems**. Oxaliplatin Injection can cause lung problems that may lead to death. Tell your doctor right away if you get any of the following symptoms as these may be indicators of a serious lung disease:
o
shortness of breath
o
wheezing
o
cough
•
**Liver problems (hepatotoxicity)**. Your doctor will do blood tests to check your liver when you start receiving Oxaliplatin Injection, and before each treatment course as needed.
•
**Heart problems**. Oxaliplatin Injection can cause heart problems that have led to death. Your doctor may do blood and heart tests during treatment with Oxaliplatin Injection if you have certain heart problems. If you faint (lose consciousness) or have an irregular heartbeat or chest pain during treatment with Oxaliplatin Injection, get medical help right away as this may be a sign of a serious heart condition.
•
**Muscle problems**. Oxaliplatin Injection can cause muscle damage (rhabdomyolysis) which can lead to death. Tell your doctor right away if you have muscle pain and swelling, along with weakness, fever, or red-brown urine.
•
**Harm to an unborn baby.** See**"****What should I tell my doctor before receiving Oxaliplatin Injection?****"**
•
**Bleeding problems (hemorrhage).** Oxaliplatin Injection when used with fluorouracil and leucovorin can cause bleeding problems (hemorrhage) that can lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs or symptoms of bleeding, including:
o
blood in your stools or black stools (looks like tar)
o
pink or brown urine
o
unexpected bleeding, or bleeding that is severe or you cannot control
o
vomit blood or vomit that looks like coffee grounds
o
cough up blood or blood clots
o
increased bruising
o
dizziness
o
weakness
o
confusion
o
changes in speech
o
headache that lasts a long time
The most common side effects of Oxaliplatin Injection include:
•
numbness, pain, tingling, and/or burning along the nerves
•
low white blood cells (blood cells important for fighting infection)
•
low platelet count (important for clotting and to control bleeding)
•
low red blood cells (blood cells that carry oxygen to the tissues)
•
nausea
•
changes in liver function tests
•
diarrhea
•
vomiting
•
tiredness
•
mouth sores
Tell your doctor if you have any side effect that bothers your or that does not go away. These are not all the possible side effects of Oxaliplatin Injection. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How can I reduce the side effects caused by cold temperatures?
•
Cover yourself with a blanket while you are getting your Oxaliplatin infusion.
•
Do not breathe deeply when exposed to cold air.
•
Wear warm clothing in cold weather at all times. Cover your mouth and nose with a scarf or a pull-down cap (ski cap) to warm the air that goes to your lungs.
•
Wear gloves when taking things from the freezer or refrigerator.
•
Drink fluids warm or at room temperature.
•
Always drink through a straw.
•
**Do not** use ice chips if you have nausea or mouth sores. Ask your doctor about what you can use.
•
Be aware that most metals are cold to touch, especially in the winter. These include your car door and mailbox. Wear gloves to touch cold objects.
•
Do not run the air-conditioning at high levels in the house or in the car in hot weather.
•
If your body gets cold, warm-up the affected part. If your hands get cold, wash them with warm water.
•
Always let your doctor know**before** your next treatment how well you did since your last visit.
Your doctor may have other useful tips for helping you with side effects.
General information about the safe and effective use of Oxaliplatin Injection
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet.
This Patient Information leaflet summarizes the most important information about Oxaliplatin Injection. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Oxaliplatin Injection that is written for health professionals.
What are the ingredients in Oxaliplatin Injection?
Active ingredient: oxaliplatin
Inactive ingredient: water for injection

For Product Inquiry:
1-800-551-7176 or www.fresenius-kabi.com/us
451427C
Revised: September 2021
This Patient Information has been approved by the U.S. Food and Drug Administration.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Oxaliplatin Injection, USP is supplied in clear, glass, single-dose vials containing 50 mg or 100 mg of oxaliplatin as a clear, colorless, sterile, preservative-free, aqueous solution at a concentration of 5 mg per mL. Water for Injection USP is present as an inactive ingredient.
|
Product Code |
Unit of Sale |
Strength |
Each |
|
775010 |
NDC 63323-750-10 |
50 mg per 10 mL (5 mg per mL) |
10 mL Single Dose Vial |
|
775020 |
NDC 63323-750-20 |
100 mg per 20 mL (5 mg per mL) |
20 mL Single Dose Vial |
The container closure is not made with natural rubber latex.
Store at 20°C to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Do not freeze and protect from light (keep in original outer carton).
Oxaliplatin is a cytotoxic drug. Follow applicable special handling and disposal procedures.1 The use of gloves is recommended. If a solution of Oxaliplatin contacts the skin, wash the skin immediately and thoroughly with soap and water. If Oxaliplatin contacts the mucous membranes, flush thoroughly with water.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of oxaliplatin. Oxaliplatin was not mutagenic to bacteria (Ames test) but was mutagenic to mammalian cells in vitro (L5178Y mouse lymphoma assay). Oxaliplatin was clastogenic both in vitro (chromosome aberration in human lymphocytes) and in vivo (mouse bone marrow micronucleus assay).
In a fertility study, male rats were given oxaliplatin at 0, 0.5, 1, or 2 mg/kg/day for five days every 21 days for a total of three cycles prior to mating with females that received two cycles of oxaliplatin on the same schedule. A dose of 2 mg/kg/day (less than one-seventh the recommended human dose on a body surface area basis) did not affect pregnancy rate, but resulted in 97% postimplantation loss (increased early resorptions, decreased live fetuses, decreased live births) and delayed growth (decreased fetal weight).
Testicular damage, characterized by degeneration, hypoplasia, and atrophy, was observed in dogs administered oxaliplatin at 0.75 mg/kg/day (approximately one-sixth of the recommended human dose on a body surface area basis) x 5 days every 28 days for three cycles. A no effect level was not identified.
