Sodium Sulfacetamide
SODIUM SULFACETAMIDE 10% CLEANSING GEL
c7b051aa-a1c4-4f0e-9278-2387155857b0
HUMAN PRESCRIPTION DRUG LABEL
Sep 9, 2025
Acella Pharmaceuticals, LLC
DUNS: 825380939
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sodium Sulfacetamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - 12 fl. oz. (355 mL) bottle
NDC 42192-146-12
Sodium Sulfacetamide
** 10%**Cleansing Gel
Rx Only
For External Use Only
12 fl. oz. (355 mL)
Acella
** PHARMACEUTICALS, LLC**

SPL UNCLASSIFIED SECTION
Note: Protect from freezing and excessive heat. The product may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Keep container or packet tightly closed.
A slight yellowish discoloration may occur on occasion when an excessive amount of the product is used and comes in contact with white fabrics. This discoloration is easily removed by normal laundering; bleaching is not necessary.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please**NOTE: This is not an Orange Book product and has not been subjected to FDA therapeutic or other equivalency testing. No representation is made as to generic status or bioequivalency.**Each person recommending a prescription substitution using this product shall make such recommendation based on his/her professional knowledge and opinion, upon evaluating the active ingredients, inactive ingredients, excipients and chemical information provided herein.
Manufactured for:
Acella Pharmaceuticals, LLC
11675 Great Oaks Way
Alpharetta, GA 30022
1-800-541-4802
Rev. 0914
DESCRIPTION SECTION
**DESCRIPTION:**Each mL of SODIUM SULFACETAMIDE 10% CLEANSING GEL contains 100 mg of sodium sulfacetamide, USP in a formulation containing Citric acid, Cocamidopropyl Betaine, Disodium EDTA, Glycerin, Glyceryl Stearate, Methylparaben, PEG-6 Caprylic/Capric Glycerides, PEG-60 Almond Glycerides, PEG-150 Pentaerythrityl Tetrastearate, Polysorbate 60, Sodium Lauryl Sulfate, Sodium Thiosulfate, Water, Xanthan Gum.
Sodium sulfacetamide is C 8H 9N 2NaO 3S·H 2O with molecular weight of 254.24. Chemically, it is N-[(4-aminophenyl)sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
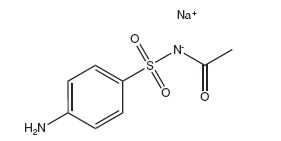
Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether.
WARNINGS SECTION
**WARNINGS:**Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug-induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome.KEEP OUT OF THE REACH OF CHILDREN.
OVERDOSAGE SECTION
**OVERDOSAGE:**The oral LD50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately.
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manifestations: Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center or your doctor.
