Rufinamide
These highlights do not include all the information needed to use RUFINAMIDE ORAL SUSPENSION safely and effectively. See full prescribing information for RUFINAMIDE ORAL SUSPENSION . RUFINAMIDE oral suspension Initial U.S. Approval: 2008
e2ba061a-49f0-4330-b0f0-00f2c3725625
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
Bionpharma Inc.
DUNS: 079637826
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rufinamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
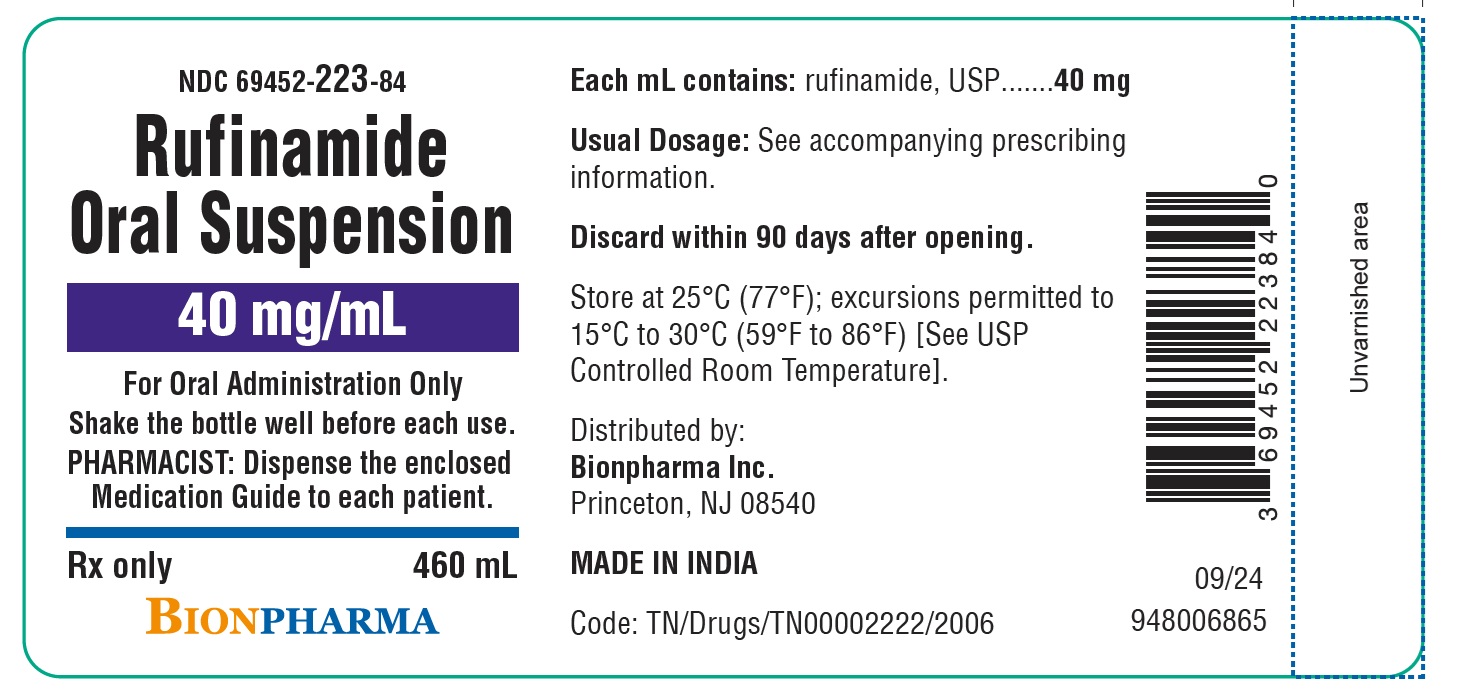
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Bottle and carton label- MADE IN INDIA
PRINCIPAL DISPLAY PANEL- 40 mg/mL ORAL SUSPENSION
NDC 69452-223-84
Rufinamide Oral Suspension
40 mg/mL
For Oral Administration Only
This product is an oral suspension liquid and is supplied with two 20 mL oral syringes for administration.
Shake the bottle well before each use
Please see instructions for administration in the enclosed full prescribing information.
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
Rx only
460 mL
BIONPHARMA

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including rufinamide, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo- treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as 1 week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1: Absolute and Relative Risk of Suicidal Behavior and Ideation|
Indication |
Placebo Patients with Events |
Drug Patients |
Relative Risk: |
Risk Difference: Additional Drug Patients with Events Per 1,000 Patients |
|
Epilepsy |
1.0 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1.0 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing rufinamide or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.2 Central Nervous System Reactions
Use of rufinamide has been associated with central nervous system-related
adverse reactions in the controlled clinical trial of patients 4 years or
older with Lennox-Gastaut Syndrome. The most significant of these can be
classified into two general categories: 1) somnolence or fatigue, and 2)
coordination abnormalities, dizziness, gait disturbances, and ataxia.
Somnolence was reported in 24% of rufinamide-treated patients compared to 13%
of patients on placebo, and led to study discontinuation in 3% of rufinamide-
treated patients compared to 0% of patients on placebo. Fatigue was reported
in 10% of rufinamide-treated patients compared to 8% of patients on placebo.
It led to study discontinuation in 1% of rufinamide-treated patients and 0% of
patients on placebo.
Dizziness was reported in 2.7% of rufinamide-treated patients compared to 0%
of patients on placebo, and did not lead to study discontinuation.
Ataxia and gait disturbance were reported in 5.4% and 1.4% of rufinamide-
treated patients, respectively, compared to no patient on placebo. None of
these reactions led to study discontinuation.
Accordingly, patients should be advised not to drive or operate machinery
until they have gained sufficient experience on rufinamide to gauge whether it
adversely affects their ability to drive or operate machinery.
5.3 QT Shortening
Formal cardiac ECG studies demonstrated shortening of the QT interval (mean = 20 msec, for doses ≥2,400 mg twice daily) with rufinamide. In a placebo- controlled study of the QT interval, a higher percentage of rufinamide-treated subjects (46% at 2,400 mg, 46% at 3,200 mg, and 65% at 4,800 mg) had a QT shortening of greater than 20 msec at T max compared to placebo (5 to 10%).
Reductions of the QT interval below 300 msec were not observed in the formal QT studies with doses up to 7,200 mg per day. Moreover, there was no signal for drug-induced sudden death or ventricular arrhythmias.
The degree of QT shortening induced by rufinamide is without any known clinical risk. Familial Short QT syndrome is associated with an increased risk of sudden death and ventricular arrhythmias, particularly ventricular fibrillation. Such events in this syndrome are believed to occur primarily when the corrected QT interval falls below 300 msec. Non-clinical data also indicate that QT shortening is associated with ventricular fibrillation.
Patients with Familial Short QT syndrome should not be treated with rufinamide. Caution should be used when administering rufinamide with other drugs that shorten the QT interval [seeContraindications(4)].
5.4 Multi-organ Hypersensitivity/Drug Reaction with Eosinophilia and
Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity, has been reported in patients taking antiepileptic drugs, including rufinamide. DRESS may be fatal or life- threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. Because this disorder is variable in its expression, other organ systems not noted here may be involved.
All cases of DRESS identified in clinical trials with rufinamide occurred in pediatric patients less than 12 years of age, occurred within 4 weeks of treatment initiation, and resolved or improved with rufinamide discontinuation. DRESS has also been reported in adult and pediatric patients taking rufinamide in the postmarketing setting.
If DRESS is suspected, the patient should be evaluated immediately, rufinamide should be discontinued, and alternative treatment should be started.
5.5 Withdrawal of AEDs
As with all antiepileptic drugs, rufinamide should be withdrawn gradually to minimize the risk of precipitating seizures, seizure exacerbation, or status epilepticus. If abrupt discontinuation of the drug is medically necessary, the transition to another AED should be made under close medical supervision. In clinical trials, rufinamide discontinuation was achieved by reducing the dose by approximately 25% every 2 days.
5.6 Status Epilepticus
Estimates of the incidence of treatment emergent status epilepticus among patients treated with rufinamide are difficult because standard definitions were not employed. In a controlled Lennox-Gastaut Syndrome trial, 3 of 74 (4.1%) rufinamide-treated patients had episodes that could be described as status epilepticus in the rufinamide-treated patients compared with none of the 64 patients in the placebo-treated patients. In all controlled trials that included patients with different epilepsies, 11 of 1,240 (0.9%) rufinamide- treated patients had episodes that could be described as status epilepticus compared with none of 635 patients in the placebo-treated patients.
5.7 Leukopenia
Rufinamide has been shown to reduce white cell count. Leukopenia (white cell count < 3X10 9L) was more commonly observed in rufinamide-treated patients 43 of 1,171 (3.7%) than placebo-treated patients, 7 of 579 (1.2%) in all controlled trials.
- Monitor patients for new or worsening depression, suicidal thoughts/behavior, and unusual changes in mood or behavior ( 5.1)
- Central nervous system reactions can occur ( 5.2)
- Use caution when administering rufinamide with other drugs that shorten the QT interval ( 5.3)
- Discontinue rufinamide if multi-organ hypersensitivity reaction occurs ( 5.4)
- Withdraw rufinamide gradually to minimize the risk of precipitating seizures, seizure exacerbation, or status epilepticus ( 5.5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
-
Suicidal Behavior and Ideation [see Warnings and Precautions (5.1)]
-
Central Nervous System Reactions [see Warnings and Precautions (5.2)]
-
QT Shortening [see Warnings and Precautions (5.3)]
-
Multi-Organ Hypersensitivity/Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.4)]
-
Leukopenia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions inAdult and Pediatric Patients ages3to 17 years of age
In the pooled, double-blind, adjunctive therapy studies in adult and pediatric patients ages 3 to 17 years of age, the most common (≥10%) adverse reactions in rufinamide-treated patients, in all doses studied (200 mg to 3,200 mg per day) with a higher frequency than in patients on placebo were: headache, dizziness, fatigue, somnolence, and nausea.
Table 2 lists adverse reactions that occurred in at least 3% of pediatric patients (ages 3 to less than 17 years) with epilepsy treated with rufinamide in controlled adjunctive studies and were numerically more common in patients treated with rufinamide than in patients on placebo.
At the target dose of 45 mg/kg per day for adjunctive therapy in pediatric patients (ages 3 to less than 17 years), the most common (≥3%) adverse reactions with an incidence greater than in placebo for rufinamide were somnolence, vomiting, and headache.
Table 2: Adverse Reactions in Pediatric Patients (Ages 3 to less than 17 years) in Pooled Double-Blind Adjunctive Trials|
Adverse Reaction |
Rufinamide |
Placebo |
|
Somnolence |
17 |
9 |
|
Vomiting |
17 |
7 |
|
Headache |
16 |
8 |
|
Fatigue |
9 |
8 |
|
Dizziness |
8 |
6 |
|
Nausea |
7 |
3 |
|
Influenza |
5 |
4 |
|
Nasopharyngitis |
5 |
3 |
|
Decreased Appetite |
5 |
2 |
|
Rash |
4 |
2 |
|
Ataxia |
4 |
1 |
|
Diplopia |
4 |
1 |
|
Bronchitis |
3 |
2 |
|
Sinusitis |
3 |
2 |
|
Psychomotor Hyperactivity |
3 |
1 |
|
Upper Abdominal Pain |
3 |
2 |
|
Aggression |
3 |
2 |
|
Ear Infection |
3 |
1 |
|
Disturbance in Attention |
3 |
1 |
|
Pruritis |
3 |
0 |
Table 3 lists adverse reactions that occurred in at least 3% of adult patients with epilepsy treated with rufinamide (up to 3,200 mg per day) in adjunctive controlled studies and were numerically more common in patients treated with rufinamide than in patients on placebo. In these studies, either rufinamide or placebo was added to the current AED therapy.
At all doses studied of up to 3,200 mg per day given as adjunctive therapy in adults, the most common (≥ 3%) adverse reactions, and with the greatest increase in incidence compared to placebo, for rufinamide were dizziness, fatigue, nausea, diplopia, vision blurred, and ataxia.
Table 3: Adverse Reactions in Adults in Pooled Double-Blind Adjunctive Trials|
Adverse Reaction |
Rufinamide |
Placebo |
|
Headache |
27 |
26 |
|
Dizziness |
19 |
12 |
|
Fatigue |
16 |
10 |
|
Nausea |
12 |
9 |
|
Somnolence |
11 |
9 |
|
Diplopia |
9 |
3 |
|
Tremor |
6 |
5 |
|
Nystagmus |
6 |
5 |
|
Blurred Vision |
6 |
2 |
|
Vomiting |
5 |
4 |
|
Ataxia |
4 |
0 |
|
Upper Abdominal Pain |
3 |
2 |
|
Anxiety |
3 |
2 |
|
Constipation |
3 |
2 |
|
Dyspepsia |
3 |
2 |
|
Back Pain |
3 |
1 |
|
Gait Disturbance |
3 |
1 |
|
Vertigo |
3 |
1 |
Discontinuation in Controlled Clinical Studies
In controlled, double-blind, adjunctive clinical studies, 9% of pediatric and adult patients receiving rufinamide as adjunctive therapy and 4% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of rufinamide (>1%) used as adjunctive therapy were generally similar in adults and pediatric patients.
In pediatric patients (ages 4 to less than 17 years) double-blind adjunctive clinical studies, 8% of patients receiving rufinamide as adjunctive therapy (at the recommended dose of 45 mg/kg per day) and 2% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of rufinamide (>1%) used as adjunctive therapy are presented in Table 4.
Table 4: Most Common Adverse Reactions Leading to Discontinuation in Pediatric Patients (Ages 4 to less than 17 years) in Pooled Double-Blind Adjunctive Trials|
Adverse****Reaction |
Rufinamide |
Placebo |
|
Convulsion |
2 |
1 |
|
Rash |
2 |
1 |
|
Fatigue |
2 |
0 |
|
Vomiting |
1 |
0 |
In adult double-blind, adjunctive clinical studies, 10% of patients receiving rufinamide as adjunctive therapy (at doses up to 3,200 mg per day) and 6% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of rufinamide (>1%) used as adjunctive therapy are presented in Table 5.
Table 5: Most Common Adverse Reactions Leading to Discontinuation in Adult Patients in Pooled Double-Blind Adjunctive Trials|
Adverse Reaction |
Rufinamide |
Placebo |
|
Dizziness |
3 |
1 |
|
Fatigue |
2 |
1 |
|
Headache |
2 |
1 |
|
Nausea |
1 |
0 |
|
Ataxia |
1 |
0 |
PediatricPatientsages1toless than 4 years
In a multi-center, parallel group, open-label study comparing rufinamide (45 mg/kg per day) adjunctive treatment (n=25) to the adjunctive treatment with an AED of the investigator’s choice (n=11) in pediatric patients (1 year to less than 4 years of age) with inadequately controlled Lennox-Gastaut Syndrome, the adverse reaction profile was generally similar to that observed in adults and pediatric patients 4 years of age and older treated with rufinamide. Adverse reactions that occurred in at least 2 (8 %) rufinamide-treated patients and with a higher frequency than in the AED comparator group were: vomiting (24%), somnolence (16%), bronchitis (12%), constipation (12%), cough (12%), decreased appetite (12%), rash (12%), otitis media (8%), pneumonia (8%), decreased weight (8%), gastroenteritis (8%), nasal congestion (8%), and pneumonia aspiration (8%).
Other AdverseReactionsObserved During Clinical Trials
Rufinamide has been administered to 1,978 individuals during all epilepsy clinical trials (placebo-controlled and open-label). Adverse reactions occurring during these studies were recorded by the investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of patients having adverse reactions, these events were grouped into standardized categories using the MedDRA dictionary. Adverse events occurring at least three times and considered possibly related to treatment are included in the System Organ Class listings below. Terms not included in the listings are those already included in the tables above, those too general to be informative, those related to procedures, and terms describing events common in the population. Some events occurring fewer than 3 times are also included based on their medical significance. Because the reports include events observed in open-label, uncontrolled observations, the role of rufinamide in their causation cannot be reliably determined.
Events are classified by body system and listed in order of decreasing frequency as follows: frequent adverse events—those occurring in at least 1/100 patients; infrequent adverse events—those occurring in 1/100 to 1/1,000 patients; rare—those occurring in fewer than 1/1,000 patients.
Blood and Lymphatic System Disorders:Frequent: anemia. Infrequent: lymphadenopathy, leukopenia, neutropenia, iron deficiency anemia, thrombocytopenia.
Cardiac Disorders:Infrequent:bundle branch block right, atrioventricular block first degree.
Metabolic and Nutritional Disorders: Frequent:decreased appetite, increased appetite.
Renal and Urinary Disorders:Frequent:pollakiuria. Infrequent:urinary incontinence, dysuria, hematuria, nephrolithiasis, polyuria, enuresis, nocturia, incontinence.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of rufinamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: Stevens-Johnson syndrome and other serious skin rashes with mucosal involvement.
Most common adverse reactions (≥ 10% and greater than placebo) were headache, dizziness, fatigue, somnolence, and nausea ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bionpharma Inc. at 1-888-235-BION or 1-888-235-2466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INSTRUCTIONS FOR USE SECTION
Instructions for Use
Rufinamide (roo fin’ a mide)
Oral Suspension
Read the Instructions for Use before using rufinamide oral suspension and each time you get a refill. There may be new information. This leaflet does not take the place of talking with the doctor about your medical condition or treatment.
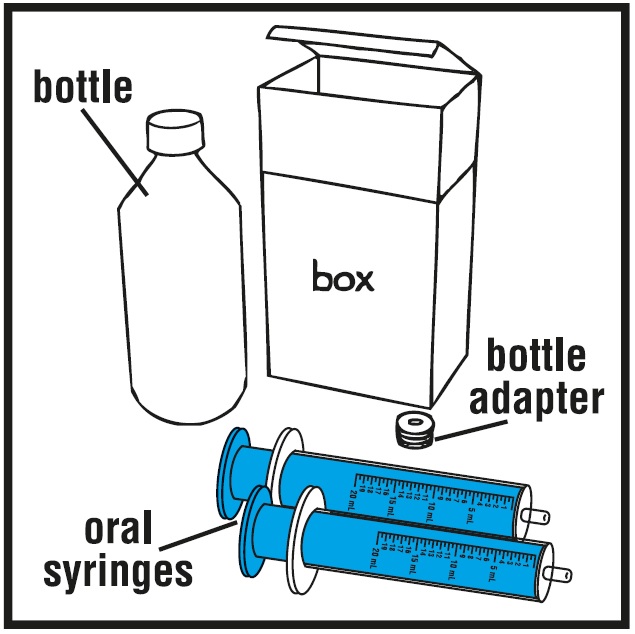
Prepare the rufinamide oral suspension dose
You will need the following supplies:See Figure A
- Rufinamide oral suspension bottle
- Bottle adapter
- Dosing oral syringe (2 dosing oral syringes are included in the rufinamide oral suspension box)
|
Yourtotal daily dose of rufinamide oral suspension is______mL. Take rufinamide oral suspension** in 2 equally divided doses:** Morning dose = _____mL** Evening dose =______mL** Note: The doctor may change your dose, especially when youarefirst starting rufinamide oral suspension**.** If your morning and evening doses are more than 20 mL each, measure each dose using either: *2 oral syringes, or *1 oral syringe, taking two steps to draw up the medicine in that same oral syringe |
**Step 1.**Remove the rufinamide oral suspension bottle, bottle adapter, and 2 oral syringes from the box.See Figure A
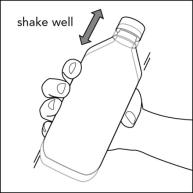
**Step 2.**Shake the bottle well before each use.See Figure B
**Step 3.**Uncap the bottle and insert the bottle adapter into the bottle. See Figure C
Once the bottle adapter is installed, it cannot be removed.See Figure D
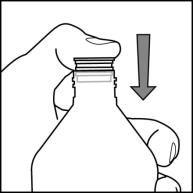
**Step 4.**Check the morning or evening dose in milliliters (mL) as prescribed by your doctor. Locate this number on the oral syringe.See Figure E
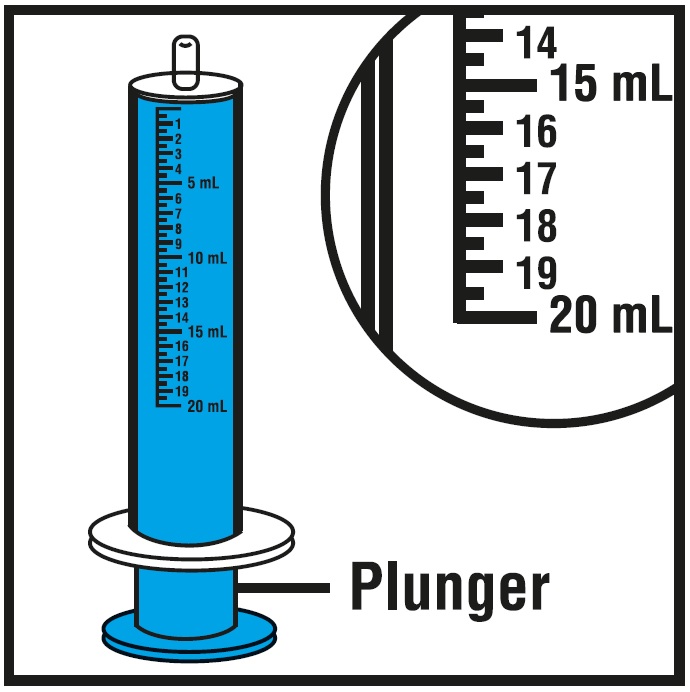
**Step 5.**Insert the oral syringe into the upright bottle and push the plunger all the way down.See Figure F
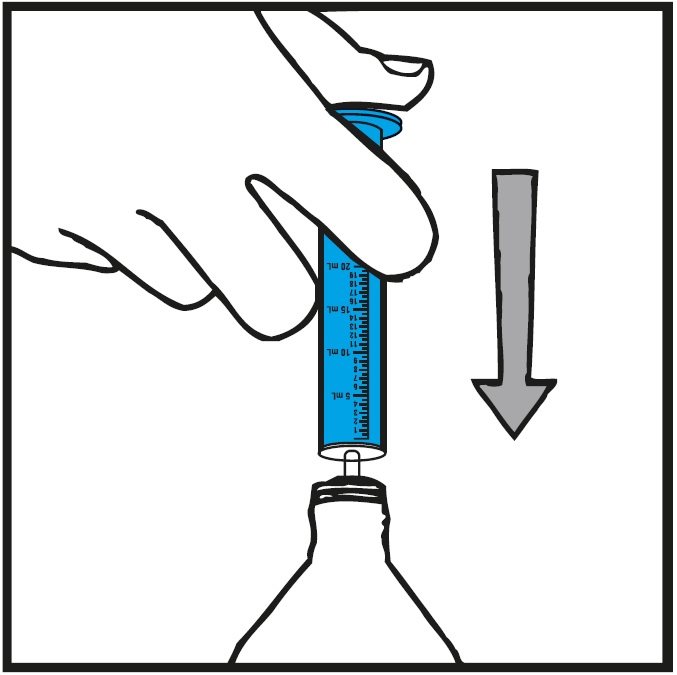
**Step 6.**With the oral syringe in place, turn the bottle upside down. Pull the plunger to the number of mL needed (the amount of liquid medicine in Step 4).See Figure G
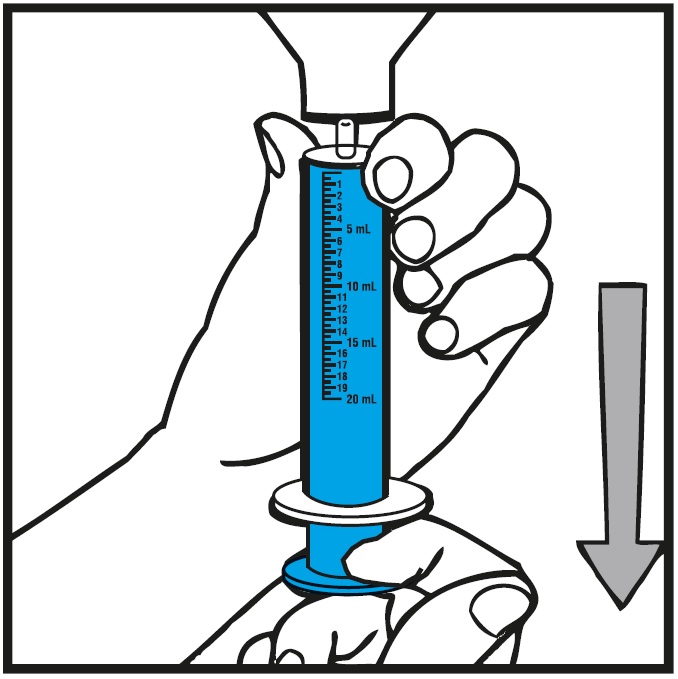
Measure the mLs of medicine using the blue plunger.See Figure H
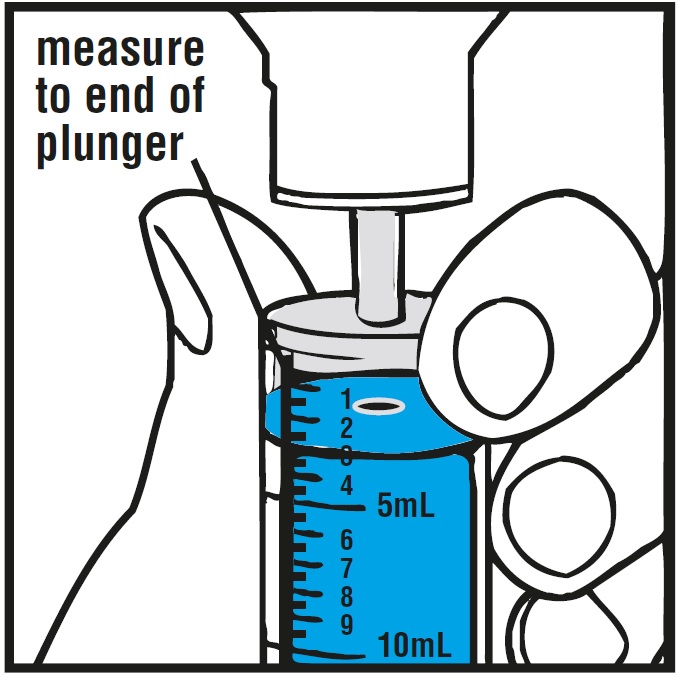
**Step 7.**If the dose is more than 20 mL, you can either use:
• 2 oral syringes, or
• 1 oral syringe, taking two steps to draw up the medicine in that same oral syringe
For example:
If your dose is 30 mL, draw up 20 mL in the first oral syringe and the remaining 10 mL in the second oral syringe.
or
If your dose is 30 mL, draw up 20 mL in the single oral syringe and squirt the medicine into your mouth, then draw up the remaining 10 mL in that same oral syringe.
Repeat Steps 4 through 6 when drawing up the remaining dose of medicine, if your dose is more than 20 mL.
**Step 8.**Remove the oral syringe from the bottle adapter.
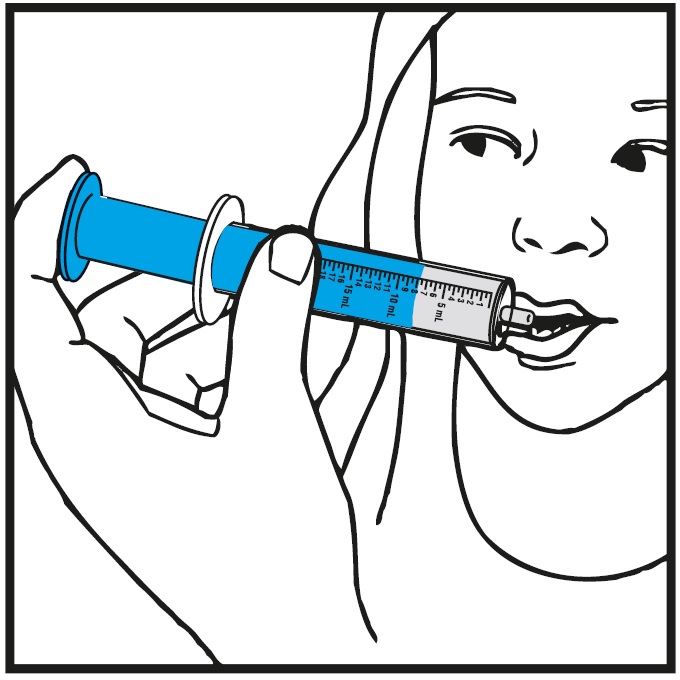
**Step 9.**Slowly squirt rufinamide oral suspension directly into the corner of your mouth. If you need 2 oral syringes for your dose, slowly squirt the medicine from the first oral syringe into your mouth, then slowly squirt the medicine from the second oral syringe into your mouth.See Figure I
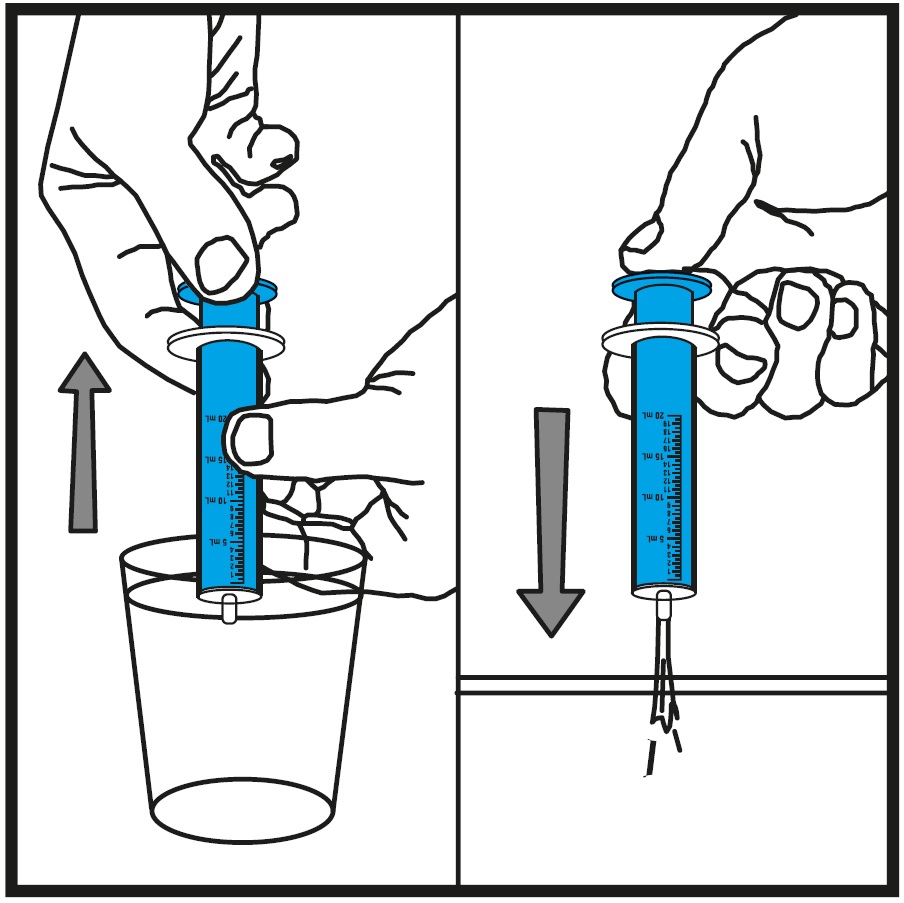
**Step 10.**Rinse the oral syringe (or oral syringes) with tap water after each use.See Figure J
- Fill a cup with water
- Pull back on the plunger and draw the water from the cup into the oral syringe
- Push on the plunger to release the water into the sink
**Step 11.**Cap the bottle tightly. The cap will fit over the bottle adapter. Store the bottle upright at 59°F to 86°F (15° to 30°C).See****Figure K
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
March 2023
FDA-05
For India manufacturing site:
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
September 2024
FDA-06
DESCRIPTION SECTION
11 DESCRIPTION
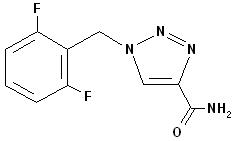
Rufinamide, USP is a triazole derivative structurally unrelated to currently marketed antiepileptic drugs (AEDs). Rufinamide, USP has the chemical name 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-triazole-4carboxamide. It has a molecular formula of C 10H 8F 2N 4O and a molecular weight of 238.2. The drug substance is a white, crystalline powder. Rufinamide, USP is insoluble in water, slightly soluble in tetrahydrofuran and in methanol, and very slightly soluble in alcohol and in acetonitrile.
Rufinamide is available for oral administration as a liquid containing rufinamide, USP at a concentration of 40 mg/mL. Inactive ingredients include anhydrous citric acid powder, hydrochloric acid, hydroxyethylcellulose, methylparaben, microcrystalline cellulose & carboxymethylcellulose sodium, natural and artificial orange flavor, poloxamer 188, potassium sorbate, propylene glycol, propylparaben, purified water, simethicone emulsion 30%, sodium hydroxide and sorbitol solution 70%.
SPL MEDGUIDE SECTION
Medication Guide
Rufinamide (roo fin’ a mide)
Oral Suspension
Read this Medication Guide before you start taking rufinamide oral suspension and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about rufinamide oral suspension?
Do not stop taking rufinamide oral suspension without first talking to your healthcare provider.
Stopping rufinamide oral suspension****suddenly can cause serious problems.
Rufinamide oral suspension can cause serious side effects, including:
**1.**Like other antiepileptic drugs, rufinamide oral suspension may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop rufinamide oral suspension****without first talking to a healthcare provider.
- Stopping rufinamide oral suspension suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
**2.**Rufinamide oral suspension may cause you to feel sleepy, tired, weak, dizzy, or have problems with coordination and walking.
What is rufinamide oral suspension?
Rufinamide oral suspension is a prescription medicine used with other medicines to treat seizures associated with Lennox-Gastaut Syndrome (LGS) in adults and pediatric patients 1 year of age and older.
It is not known if rufinamide oral suspension is safe and effective in the treatment of Lennox-Gastaut Syndrome in pediatric patients under 1 year of age.
Who should not take rufinamide oral suspension?
Do not take rufinamide oral suspension if you have a genetic condition called familial short QT syndrome, a problem that affects the electrical system of the heart.
What should I tell my healthcare provider before taking rufinamide oral suspension?
Before you take rufinamide oral suspension, tell your healthcare provider if you:
-
have heart problems
-
have liver problems
-
have any other medical problems
-
have or have had suicidal thoughts or actions, depression or mood problems
-
are pregnant or plan to become pregnant. It is not known if rufinamide oral suspension can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking rufinamide oral suspension. You and your healthcare provider will decide if you should take rufinamide oral suspension while you are pregnant.
-
Rufinamide oral suspension may make certain types of birth control less effective. Talk to your healthcare provider about the best birth control methods for you while you take rufinamide oral suspension.
○ If you become pregnant while taking rufinamide oral suspension, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic medicines during pregnancy. -
are breastfeeding or plan to breastfeed. It is not known if rufinamide will pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take rufinamide oral suspension.
**Tell your healthcare provider about all the medicines you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking rufinamide oral suspension with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I take rufinamide oral suspension?
- Take rufinamide oral suspension exactly as your healthcare provider tells you. Your healthcare provider will tell you how much rufinamide oral suspension to take.
- Your healthcare provider may change your dose. Do not change your dose of rufinamide oral suspension without talking to your healthcare provider.
- Take rufinamide oral suspension with food.
- If you take rufinamide oral suspension, shake the bottle well before you take each dose. Measure your dose of rufinamide oral suspension using the bottle adapter and dosing syringes provided.
See the completeInstructions for Usebelow for information on how to use the dosing syringes and measure your dose of rufinamide oral suspension.
- If you take too much rufinamide oral suspension, call your local Poison Control Center or get emergency medical help right away.
What should I avoid while taking rufinamide oral suspension?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking rufinamide oral suspension until you talk to your healthcare provider. Rufinamide oral suspension taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how rufinamide oral suspension affects you. Rufinamide oral suspension can slow your thinking and motor skills.
What are the possible side effects of rufinamide oral suspension?
See “What is the most important information I should know about rufinamide oral suspension?”
Rufinamide oral suspension may cause serious side effects including:
- Rufinamide oral suspension can also cause allergic reactions or serious problems which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
Call your healthcare provider right away if you have****any of the following. Symptoms may include:
- swelling of your face, eyes, lips, or tongue
- trouble swallowing or breathing
- a skin rash
- hives
- fever, swollen glands, or sore throat that do not go away or come and go
- swollen glands
- yellowing of your skin or eyes
- dark urine
- unusual bruising or bleeding
- severe fatigue or weakness
- severe muscle pain
- your seizures happen more often or become worse
Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of rufinamide oral suspension include:
- headache
- dizziness
- tiredness
- sleepiness
- nausea
- vomiting
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of rufinamide oral suspension. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store rufinamide oral suspension?
-
Store rufinamide oral suspension at 59°F to 86°F (15°C to 30°C).
-
Replace the cap securely after opening.
-
Keep rufinamide oral suspension in an upright position.
-
Use rufinamide oral suspension within 90 days of first opening the bottle.
Keep rufinamide oral suspension and all medicines out of the reach of children.
General Information about the safe and effective use of rufinamide oral suspension
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use rufinamide oral suspension for a condition for which it was not prescribed. Do not give rufinamide oral suspension to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about rufinamide oral suspension. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about rufinamide oral suspension that is written for health professionals.
For more information, call 1-888-235-BION or 1-888-235-2466.
What are the ingredients in rufinamide oral suspension?
Active ingredient: rufinamide, USP
Inactive ingredients: anhydrous citric acid powder, hydrochloric acid, hydroxyethylcellulose, methylparaben, microcrystalline cellulose & carboxymethylcellulose sodium, natural and artificial orange flavor, poloxamer 188, potassium sorbate, propylene glycol, propylparaben, purified water, simethicone emulsion 30%, sodium hydroxide and sorbitol solution 70%.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
Revised: March 2024
This Medication Guide has been approved by the U.S. Food and Drug Administration.
FDA-07
For India manufacturing site:
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
Revised: September 2024
This Medication Guide has been approved by the U.S. Food and Drug
Administration.
FDA-08
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Oral Suspension: 40 mg/mL. White to off-white, orange flavored liquid.
- Oral suspension: 40 mg/mL ( 3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as rufinamide, during pregnancy. Encourage women who are taking rufinamide during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org.
Risk Summary
There are no adequate data on the developmental risks associated with use of rufinamide in pregnant women. In animal reproduction studies, oral administration of rufinamide resulted in developmental toxicity in pregnant rats and rabbits at clinically relevant doses [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal data
Oral administration of rufinamide (0, 20, 100, or 300 mg/kg/day) to pregnant rats throughout organogenesis resulted in decreased fetal weight and increased incidence of fetal skeletal abnormalities at 100 and 300 mg/kg/day, which were associated with maternal toxicity. The maternal plasma exposure (AUC) at the no-adverse effect dose (20 mg/kg/day) for developmental toxicity was less than that in humans at the maximum recommended human dose (MRHD) of 3,200 mg/day.
Oral administration of rufinamide (0, 30, 200, or 1,000 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in embryofetal death, decreased fetal body weight, and increased incidence of fetal visceral and skeletal abnormalities at doses of 200 and 1,000 mg/kg/day. The high dose (1,000 mg/kg/day) was associated with abortion. Plasma exposure (AUC) at the no- adverse effect dose (30 mg/kg/day) was less than that in humans at the MRHD.
When rufinamide was orally administered (0, 5, 30, or 150 mg/kg/day) to pregnant rats throughout pregnancy and lactation, decreased offspring growth and survival were observed at all doses tested. A no-effect dose for adverse effects on pre- and postnatal development was not established. At the lowest dose tested (5 mg/kg/day), plasma exposure (AUC) was less than that in humans at the MRHD.
8.2 Lactation
Risk Summary
There are no data on the presence of rufinamide in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rufinamide and any potential adverse effects on the breastfed infant from rufinamide or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Use of rufinamide may reduce the effectiveness of hormonal contraceptives containing ethinyl estradiol or norethindrone. Advise women of reproductive potential taking rufinamide who are using a contraceptive containing ethinyl estradiol and norethindrone to use an additional non-hormonal form of contraception [see Drug Interactions ( 7.3) and Clinical Pharmacology ( 12.3)] .
Infertility
The effect of rufinamide on fertility in humans has not been established. Oral administration of rufinamide (20, 60, 200, and 600 mg/kg/day) to male and female rats prior to mating, during mating, and during early gestation (females only) resulted in the impairment of fertility at all dose levels tested. The no-effect dose was not established. The plasma exposure level at 20 mg/kg was approximately 0.2 times the human plasma AUC at the MRHD [see Nonclinical Toxicology ( 13.1)].
8.4 Pediatric Use
Safety and effectiveness have been established in pediatric patients 1 to 17 years of age. The effectiveness of rufinamide in pediatric patients 4 years of age and older was based upon an adequate and well-controlled trial of rufinamide that included both adults and pediatric patients, 4 years of age and older, with Lennox-Gastaut Syndrome. The effectiveness in patients 1 to less than 4 years was based upon a bridging pharmacokinetic and safety study [see Dosage and Administration ( 2.1), Adverse Reactions ( 6.1), and Clinical Studies ( 14)]. The pharmacokinetics of rufinamide in the pediatric patients, ages 1 to less than 4 years of age is similar to children older than 4 years of age and adults [see Clinical Pharmacology ( 12.3)] .
Safety and effectiveness in pediatric patients below the age of 1 year has not been established.
Oral administration of rufinamide (0, 15, 50, or 150 mg/kg) to young rats for 10 weeks starting on postnatal day 7 resulted in decreased brain weights at the mid and high doses and neurobehavioral impairment (learning and memory deficit, altered startle response, decreased locomotor activity) and decreased growth (decreased body weight) at the highest dose tested. The no-effect dose for adverse effects on postnatal development in rats (15 mg/kg) was associated with a plasma exposure (AUC) lower than that in humans at the maximum recommended human dose (MRHD) of 3,200 mg/day.
8.5 Geriatric Use
Clinical studies of rufinamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Pharmacokinetics of rufinamide in the elderly are similar to that in the young subjects [see Clinical Pharmacology ( 12.3)] .
8.6 Renal Impairment
Rufinamide pharmacokinetics in patients with severe renal impairment (creatinine clearance < 30 mL/min) was similar to that of healthy subjects. Dose adjustment in patients undergoing dialysis should be considered [see Clinical Pharmacology(12.3)].
8.7 Hepatic Impairment
Use of rufinamide in patients with severe hepatic impairment (Child-Pugh score 10 to 15) is not recommended. Caution should be exercised in treating patients with mild (Child-Pugh score 5 to 6) to moderate (Child-Pugh score 7 to 9) hepatic impairment.
- Pregnancy: Based on animal data, may cause fetal harm. ( 8.1)
- Renal impairment: Consider adjusting the rufinamide dose for the loss of drug upon dialysis ( 8.6)
- Not recommended in patients with severe hepatic impairment ( 8.7)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Administration Information
- Advise patients to take rufinamide oral suspension with food [see Dosage and Administration (2.2)].
- Advise patients who are prescribed the oral suspension to shake the bottle vigorously before every administration and to use the adaptor and oral dosing syringe [see Dosage and Administration (2.2)].
Suicidal Thinking and Behavior
Inform patients, their caregivers, and families that antiepileptic drugs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers [see Warnings and Precautions (5.1)].
Central Nervous System Reactions
Inform patients about the potential for somnolence or dizziness and advise them not to drive or operate machinery until they have gained sufficient experience on rufinamide oral suspension to gauge whether it adversely affects their mental and/or motor performance [see Warnings and Precautions (5.2)].
Multi-Organ Hypersensitivity Reactions
Advise patients to notify their physician if they experience a rash associated with fever [see Warnings and Precautions (5.4)].
Drug Interactions
- Inform female patients of childbearing age that the concurrent use of rufinamide oral suspension with hormonal contraceptives may render this method of contraception less effective. Recommend patients use additional non-hormonal forms of contraception when using rufinamide oral suspension [see Drug Interactions (7.3) and Use in Specific Populations ( 8.3)] .
- Inform patients that alcohol in combination with rufinamide oral suspension may cause additive central nervous system effects.
Pregnancy
Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. To enroll, patients can call the toll-free number 1-888-233-2334 [seeUse in Specific Populations (8.1)].
Breast-feeding
Advise patients to notify their physician if they are breast-feeding or intend to breast-feed [see Use in Specific Populations (8.2)].
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
Revised: March 2024
FDA-07
For India Manufacturing site:
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
Revised: September 2024
FDA-08
948026851
