Droxidopa
DROXIDOPA CAPSULES. These highlights do not include all the information needed to use DROXIDOPA CAPSULES safely and effectively. See full prescribing information for DROXIDOPA CAPSULES. DROXIDOPA capsules, for oral use Initial U.S. Approval: 2014
fe08aebb-b390-d176-e053-6394a90a6be7
HUMAN PRESCRIPTION DRUG LABEL
Jun 13, 2023
Golden State Medical Supply, Inc.
DUNS: 603184490
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
droxidopa
PRODUCT DETAILS
INGREDIENTS (8)
droxidopa
PRODUCT DETAILS
INGREDIENTS (9)
droxidopa
PRODUCT DETAILS
INGREDIENTS (8)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Supine Hypertension
Droxidopa therapy may cause or exacerbate supine hypertension in patients with nOH. Patients should be advised to elevate the head of the bed when resting or sleeping. Monitor blood pressure, both in the supine position and in the recommended head-elevated sleeping position. Reduce or discontinue Droxidopa if supine hypertension persists. If supine hypertension is not well-managed, Droxidopa may increase the risk of cardiovascular events, particularly stroke.
5.2 Hyperpyrexia and Confusion
Postmarketing cases of a symptom complex resembling neuroleptic malignant syndrome (NMS) have been reported with Droxidopa use during postmarketing surveillance. Observe patients carefully when the dosage of Droxidopa is changed or when concomitant levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics.
NMS is an uncommon but life-threatening syndrome characterized by fever or hyperthermia, muscle rigidity, involuntary movements, altered consciousness, and mental status changes. The early diagnosis of this condition is important for the appropriate management of these patients.
5.3 Ischemic Heart Disease, Arrhythmias, and Congestive Heart Failure
Droxidopa may exacerbate existing ischemic heart disease, arrhythmias, and congestive heart failure. Careful consideration should be given to this potential risk prior to initiating therapy in patients with these conditions.
5.4 Allergic Reactions
Hypersensitivity reactions including anaphylaxis, angioedema, bronchospasm, urticaria and rash have been reported in postmarketing experience. Some of these reactions resulted in emergency treatment. If a hypersensitivity reaction occurs, discontinue the drug and initiate appropriate therapy.
- Droxidopa may cause supine hypertension and may increase cardiovascular risk if supine hypertension is not well-managed ( 5.1)
- Hyperpyrexia and confusion ( 5.2)
- May exacerbate symptoms in patients with existing ischemic heart disease, arrhythmias, and congestive heart failure ( 5.3)
- Allergic reactions ( 5.4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs that Increase Blood Pressure
Administering Droxidopa capsules in combination with other agents that increase blood pressure (e.g., norepinephrine, ephedrine, midodrine, and triptans) would be expected to increase the risk for supine hypertension.
7.2 Parkinson's Medications
Dopa-decarboxylase inhibitors may require dose adjustments for Droxidopa.
7.3 Non-selective MAO Inhibitors
The concomitant use of selective MAO-B inhibitors, such as rasagiline or selegiline, was permitted in the Droxidopa clinical trials. However, based on mechanism of action, the use of non-selective MAO inhibitors and linezolid should be avoided as there is a potential for increased blood pressure when taken with Droxidopa.
Use of DOPA decarboxylase inhibitors may require dose adjustments for Droxidopa ( 7.2)
DESCRIPTION SECTION
11 DESCRIPTION
Droxidopa capsules contain droxidopa, which is a synthetic amino acid precursor of norepinephrine, for oral administration. Chemically, droxidopa is (–)-threo-3-(3,4- Dihydroxyphenyl)-L-serine. It has the following structural formula:
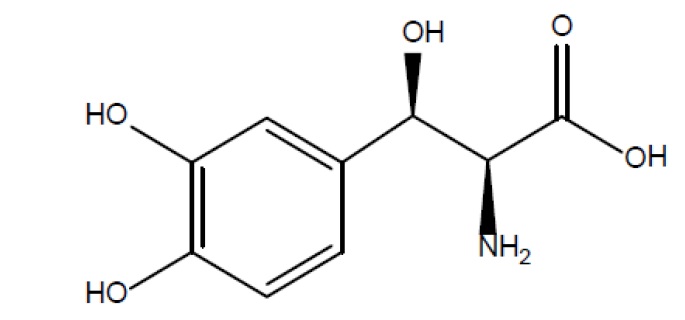
Droxidopa is a white to light brown crystalline powder. It is soluble in dilute hydrochloric acid, and practically insoluble in methanol, glacial acetic acid, ethanol, acetone, ether and chloroform. It has a molecular weight of 213.19 and a molecular formula of C 9H 11NO 5.
Droxidopa capsules also contain the following inactive ingredients: mannitol, corn starch, pregelatinized starch, and magnesium stearate. The capsule shell is printed with black ink. The black inks contain shellac, ethanol, isopropyl alcohol, n-butyl alcohol, propylene glycol, ammonia solution, iron oxide black and potassium hydroxide. The capsule shell contains the following inactive ingredients: 100 mg – gelatin, titanium dioxide, FD&C Blue No. 2, black and red iron oxide; 200 mg – gelatin, titanium dioxide, black and yellow iron oxide; 300 mg – gelatin, titanium dioxide, FD&C Blue No. 1and D&C Yellow No. 10. Droxidopa capsules differ in size and color by strength [see Dosage Forms and Strengths ( 3)] .
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies have been conducted at dosages up to 1,000 mg/kg/day in mice and up to 100 mg/kg/day in rats with no indication of carcinogenic effects. Based on dose per unit body surface area, these two doses correspond to approximately 3 and 0.5 times, respectively, the maximum recommended total daily dose of 1,800 mg in a 60 kg patient. Droxidopa was clastogenic in Chinese hamster ovary cells (chromosome aberration assay), but was not mutagenic in bacteria (Ames assay), and was not clastogenic in a mouse micronucleus assay.
Studies in rats show that droxidopa has no effect on fertility.
13.2 Animal Toxicology and/or Pharmacology
In long-term chronic toxicity studies, rats and mice treated for 52 and 80 weeks, respectively, at doses up to 300 mg/kg/day in rats and 1,000 mg/kg/day in mice had increased incidences of renal and cardiac lesions (rats and mice) and deaths (rats only). The doses at which these effects were not seen represented 0.2 and 0.3 times, in rats and mice, respectively, the maximum recommended total daily dose of 1,800 mg in a 60 kg patient, when based on body surface area.
No signs of toxicity were observed in monkeys or dogs given droxidopa for 13 weeks at doses 32 times (3,000 mg/kg/day) and 37 times (2,000 mg/kg/day), respectively, the maximum human dose.
SPL UNCLASSIFIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Droxidopa capsules are supplied in the following dosage strengths:
100 mg: Off white to light brownish powder filled in size “3” hard gelatin capsules with opaque light blue colored cap and opaque white colored body imprinted ‘SG’ on cap and ‘429’ on body with black ink.
Bottles of 90 NDC 51407-765-90
200 mg: Off white to light brownish powder filled in size “2” hard gelatin capsules with opaque light yellow colored cap and opaque white colored body imprinted ‘SG’ on cap and ‘430’ on body with black ink.
Bottles of 90 NDC 51407-766-90
300 mg: Off white to light brownish powder filled in size “1” hard gelatin capsules with opaque light green colored cap and opaque white colored body imprinted ‘SG’ on cap and ‘431’ on body with black ink.
Bottles of 90 NDC 51407-767-90
16.2 Storage and Handling
Droxidopa capsules should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [ see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Elevations in Blood Pressure
Counsel patients that Droxidopa causes elevations in blood pressure and increases the risk of supine hypertension, which could lead to strokes, heart attacks, and death. Instruct patients to rest and sleep in an upper-body elevated position and monitor blood pressure. Instruct patients how to manage observed blood pressure elevations. To reduce the risk of supine hypertension, in addition to raising the upper body, the late afternoon dose of Droxidopa should be taken at least three hours before bedtime [see Warnings and Precautions ( 5.1)] .
Concomitant Treatments
Counsel patients about the concomitant use of drugs to treat other conditions that may have an additive effect with Droxidopa [see Drug Interactions ( 7)].
Allergic Reactions
Counsel patients to discontinue Droxidopa and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction such as anaphylaxis, angioedema, bronchospasm, urticaria or rash occur [see Warnings and Precautions ( 5.4)].
Lactation
Advise women not to breastfeed during treatment with Droxidopa [see Use in Specific Populations ( 8.2)] .
Food
Patients should take Droxidopa the same way each time, either with food or without food [see Dosage and Administration ( 2.1)] .
Missed Dose
If a dose is missed, patients should take the next dose at the regularly scheduled time and should not double the dose.
Manufactured by:
ScieGen Pharmaceuticals Inc
Hauppauge, NY 11788 USA
Rev. 1/2021
Marketed by:
GSMS, Inc.
Camarillo, CA USA 93012
