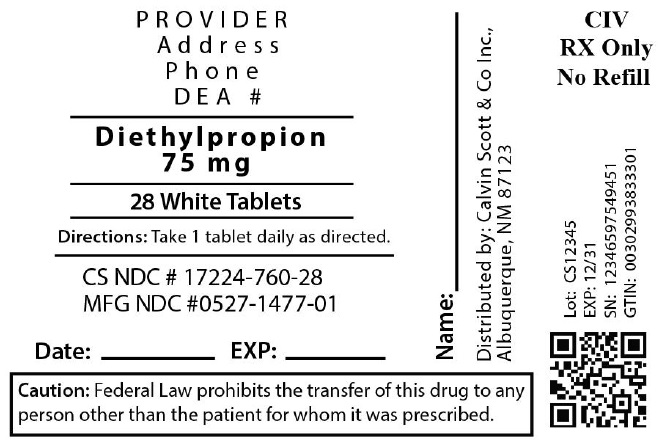
Diethylpropion Hydrochloride
Diethylpropion Hydrochloride Extended Release Tablets, 75 mg CIV Rx only
Approved
Approval ID
d6cde8cc-26ae-4087-8eac-b11192749cc5
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Mar 3, 2023
Manufacturers
FDA
Calvin Scott & Co., Inc.
DUNS: 073404626
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DIETHYLPROPION HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code17224-760
Application NumberANDA091680
Product Classification
M
Marketing Category
C73584
G
Generic Name
DIETHYLPROPION HYDROCHLORIDE
Product Specifications
Route of AdministrationORAL
Effective DateMarch 3, 2023
FDA Product Classification
INGREDIENTS (7)
MANNITOLInactive
Code: 3OWL53L36A
Classification: IACT
CARBOMER HOMOPOLYMER TYPE AInactive
Code: F68VH75CJC
Classification: IACT
TARTARIC ACIDInactive
Code: W4888I119H
Classification: IACT
HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MM2/S)Inactive
Code: 6N003M473W
Classification: IACT
POVIDONE K30Inactive
Code: U725QWY32X
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
DIETHYLPROPION HYDROCHLORIDEActive
Quantity: 75 mg in 1 1
Code: 19V2PL39NG
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 3/3/2023
Package Labeling:17224-760-28