Meclizine Hydrochloride
These highlights do not include all the information needed to use MECLIZINE HYDROCHLORIDE safely and effectively. See full prescribing information for MECLIZINE HYDROCHLORIDE. MECLIZINE hydrochloride tablets, for oral use MECLIZINE hydrochloride chewable tablets, for oral use Initial U.S. Approval: 1957
112103bc-25e2-42cf-8542-ba1d3392a2c3
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Meclizine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
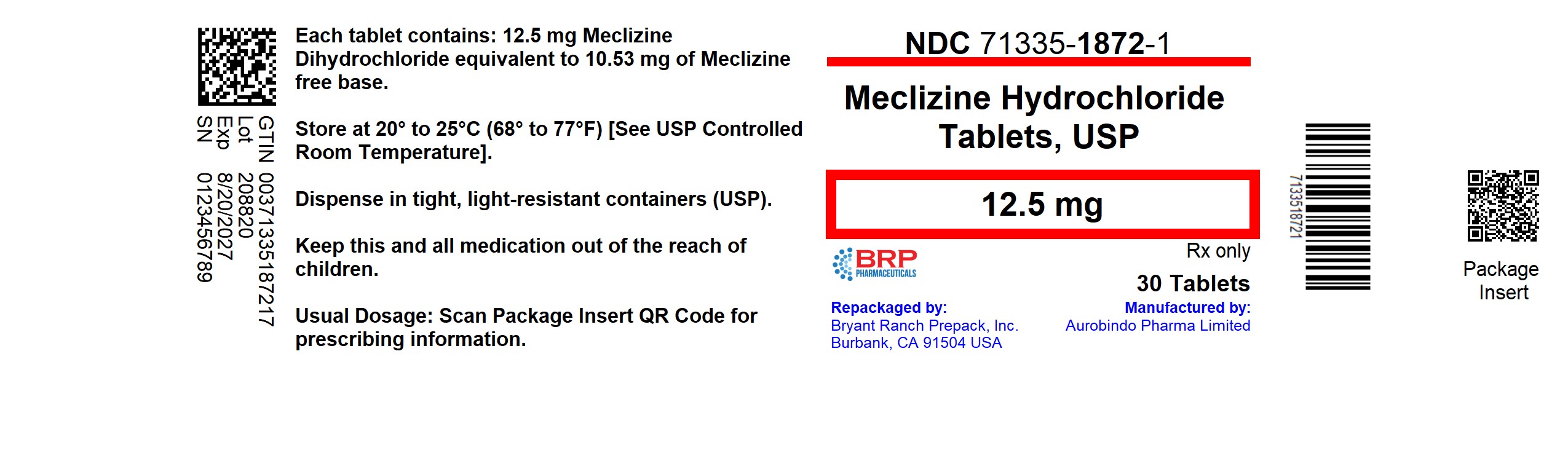
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Meclizine Hcl 12.5mg Tablet
DESCRIPTION SECTION
11 DESCRIPTION
Meclizine hydrochloride, a histamine (H1) receptor antagonist, is a white or slightly yellowish, crystalline powder. It has the following structural formula:
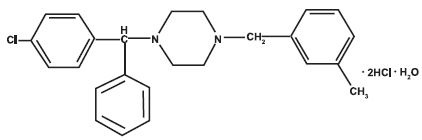
Chemically, meclizine hydrochloride is 1-(p-chloro-α- phenylbenzyl)-4-(m-methylbenzyl) piperazine dihydrochloride monohydrate.
Tablets
Inactive ingredients for the tablets are: corn starch; dibasic calcium
phosphate; magnesium stearate; polyethylene glycol; sucrose. The 12.5 mg
tablets also contain: FD&C Blue # 1. The 25 mg tablets also contain: FD&C
Yellow # 6 and D&C Yellow # 10. The 50 mg tablets also contain: FD&C Blue # 1,
FD&C Yellow # 6 and D&C Yellow # 10.
Each meclizine hydrochloride 12.5 mg tablet contains 12.5 mg of meclizine
dihydrochloride equivalent to 10.53 mg of meclizine free base.
Each meclizine hydrochloride 25 mg tablet contains 25 mg of meclizine
dihydrochloride equivalent to 21.07 mg of meclizine free base.
Each meclizine hydrochloride 50 mg tablet contains 50 mg of meclizine
dihydrochloride equivalent to 42.14 mg of meclizine free base.
Chewable Tablets
Inactive ingredients for the chewable tablets are: corn starch, colloidal
silicon dioxide, FD&C Red # 40, lactose monohydrate, magnesium stearate,
raspberry flavor, saccharin sodium, and talc.
Each meclizine hydrochloride 25 mg chewable tablet contains 25 mg of meclizine dihydrochloride equivalent to 21.07 mg of meclizine free base.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Meclizine hydrochloride 12.5 mg tablets are oval shaped, biconvex, two-layered tablet, one blue to pale blue layer debossed with “34” and one white to off white layer debossed with “L”.
NDC: 71335-1872-1: 30 Tablets in a BOTTLE
NDC: 71335-1872-2: 60 Tablets in a BOTTLE
NDC: 71335-1872-3: 90 Tablets in a BOTTLE
NDC: 71335-1872-4: 28 Tablets in a BOTTLE
NDC: 71335-1872-5: 20 Tablets in a BOTTLE
NDC: 71335-1872-6: 120 Tablets in a BOTTLE
NDC: 71335-1872-7: 100 Tablets in a BOTTLE
NDC: 71335-1872-8: 10 Tablets in a BOTTLE
Store at 20oC to 25oC (68oF to 77oF) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container (USP).
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
