Oxybutynin Chloride
OXYBUTYNIN CHLORIDE TABLETS, USP
5fcc6e9f-d5f0-4521-b6f3-315146b65f82
HUMAN PRESCRIPTION DRUG LABEL
Jun 7, 2010
State of Florida DOH Central Pharmacy
DUNS: 829348114
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
oxybutynin chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. Oxybutynin chloride exhibits only one-fifth of the anticholinergic activity of atropine on the rabbit detrusor muscle, but four to ten times the antispasmodic activity. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Oxybutynin chloride relaxes bladder smooth muscle. In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin chloride increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void. Oxybutynin chloride thus decreases urgency and the frequency of both incontinent episodes and voluntary urination.
Antimuscarinic activity resides predominately in the R-isomer. A metabolite, desethyloxybutynin, has pharmacological activity similar to that of oxybutynin in in vitro studies.
Pharmacokinetics
Absorption
Following oral administration of oxybutynin chloride, oxybutynin is rapidly absorbed achieving Cmax within an hour, following which plasma concentration decreases with an effective half-life of approximately 2 to 3 hours. The absolute bioavailability of oxybutynin is reported to be about 6% (range 1.6 to 10.9%) for the tablets. Wide interindividual variation in pharmacokinetic parameters is evident following oral administration of oxybutynin.
The mean pharmacokinetic parameters for R- and S-oxybutynin are summarized in Table 1. The plasma concentration-time profiles for R- and S-oxybutynin are similar in shape; Figure 1 shows the profile for R-oxybutynin.
Table 1|
Mean (SD) R- and S-Oxybutynin Pharmacokinetic Parameters Following Three Doses of Oxybutynin Chloride 5 mg Administered every 8 Hours (n=23) | ||
|
Parameters (units) |
R-Oxybutynin |
S-Oxybutynin |
|
Cmax (ng/mL) |
3.6 (2.2) |
7.8 (4.1) |
|
Tmax (h) |
0.89 (0.34) |
0.65 (0.32) |
|
AUCt (ng•h/mL) |
22.6 (11.3) |
35.0 (17.3) |
|
AUCinf (ng•h/mL) |
24.3 (12.3) |
37.3 (18.7) |
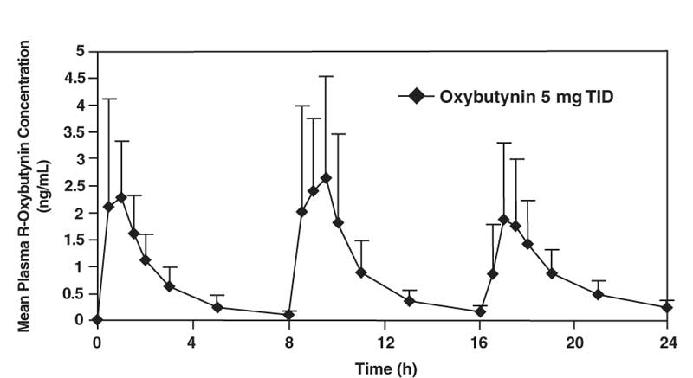
**Figure 1.**Mean R-oxybutynin plasma concentrations following three doses of oxybutynin chloride 5 mg administered every 8 hours for 1 day in 23 healthy adult volunteers
Oxybutynin chloride steady-state pharmacokinetics were also studied in 11 pediatric patients with detrusor overactivity associated with a neurological condition (e.g., spina bifida). These pediatric patients were on oxybutynin chloride tablets with total daily dose ranging from 7.5 mg to 15 mg (0.22 to 0.53 mg/kg). Overall, most patients (86.9%) were taking a total daily oxybutynin chloride dose between 10 mg and 15 mg. Sparse sampling technique was used to obtain serum samples. When all available data are normalized to an equivalent of 5 mg twice daily oxybutynin chloride, the mean pharmacokinetic parameters derived for R- and S-oxybutynin and R- and S-desethyloxybutynin are summarized in Table 2. The plasma-time concentration profiles for R- and S-oxybutynin are similar in shape; Figure 2 shows the profile for R-oxybutynin when all available data are normalized to an equivalent of 5 mg twice daily.
Table 2
| ||||
|
Mean ± SD R- and S-Oxybutynin and R- and S-Desethyloxybutynin Pharmacokinetic Parameters In Children Aged 5–15 Following Administration of 7.5 mg to 15 mg Total Daily Dose of Oxybutynin Chloride Tablets (N=11) | ||||
|
All Available Data Normalized to an Equivalent of Oxybutynin Chloride Tablets 5 mg BID or TID at Steady State | ||||
|
R-Oxybutynin |
S-Oxybutynin |
R-Desethyloxybutynin |
S-Desethyloxybutynin | |
|
Cmax* (ng/mL) |
6.1 ± 3.2 |
10.1 ± 7.5 |
55.4 ± 17.9 |
28.2 ± 10.0 |
|
Tmax (hr) |
1.0 |
1.0 |
2.0 |
2.0 |
|
AUC† (ng∙hr/mL) |
19.8 ± 7.4 |
28.4 ± 12.7 |
238.8 ± 77.6 |
119.5 ± 50.7 |
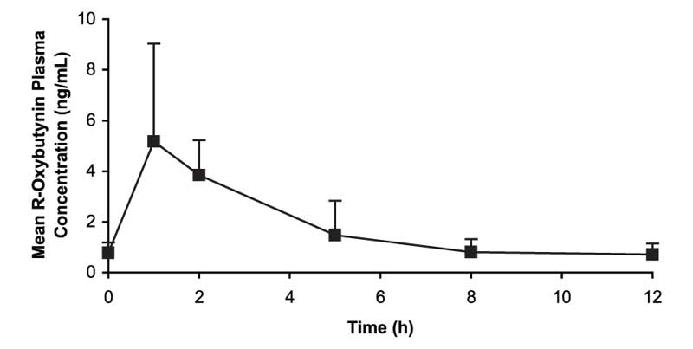
**Figure 2.**Mean steady-state (±SD) R-oxybutynin plasma concentrations following administration of total daily oxybutynin chloride tablet dose of 7.5 mg to 15 mg (0.22 mg/kg to 0.53 mg/kg) in children 5‑15 years of age. – Plot represents all available data normalized to the equivalent of oxybutynin chloride 5 mg BID or TID at steady state
Food Effects
Data in the literature suggests that oxybutynin solution co-administered with food resulted in a slight delay in absorption and an increase in its bioavailability by 25% (n=18).1
Distribution
Plasma concentrations of oxybutynin decline biexponentially following intravenous or oral administration. The volume of distribution is 193 L after intravenous administration of 5 mg oxybutynin chloride.
Metabolism
Oxybutynin is metabolized primarily by the cytochrome P450 enzyme systems, particularly CYP3A4 found mostly in the liver and gut wall. Its metabolic products include phenylcyclohexylglycolic acid, which is pharmacologically inactive, and desethyloxybutynin, which is pharmacologically active.
Excretion
Oxybutynin is extensively metabolized by the liver, with less than 0.1% of the administered dose excreted unchanged in the urine. Also, less than 0.1% of the administered dose is excreted as the metabolite desethyloxybutynin.
