sorafenib
These highlights do not include all the information needed to use SORAFENIB TABLETS safely and effectively. See full prescribing information for SORAFENIB TABLETS. SORAFENIB tablets, for oral use Initial U.S. Approval: 2005
93f9c3b2-d4c5-80d3-5421-8b4c29fbf8a6
HUMAN PRESCRIPTION DRUG LABEL
Nov 18, 2022
Dr.Reddys Laboratories Inc
DUNS: 802315887
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
sorafenib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Events
In the SHARP (HCC) study, the incidence of cardiac ischemia/infarction was 2.7% in sorafenib-treated patients compared with 1.3% in those receiving placebo; in the TARGET (RCC) study, the incidence of cardiac ischemia/infarction was higher in the sorafenib-treated group (2.9%) compared with patients receiving placebo (0.4%), and in the DECISION (DTC) study, the incidence of cardiac ischemia/infarction was 1.9% in the sorafenib-treated group compared with 0% in patients receiving placebo. Patients with unstable coronary artery disease or recent myocardial infarction were excluded from this study. In multiple clinical trials, congestive heart failure has been reported in 1.9% of sorafenib-treated patients (N=2276) [see Adverse Reactions (6.2)].
Consider temporary or permanent discontinuation of sorafenib tablets in patients who develop cardiovascular events [see Dosage and Administration (2.2)].
5.2 Hemorrhage
An increased risk of bleeding may occur following sorafenib tablets administration. In the SHARP (HCC) study, the rates of bleeding from esophageal varices (2.4% and 4%) and of bleeding with a fatal outcome from any site (2.4% and 4%) were similar in sorafenib-treated patients and those receiving placebo, respectively. In the TARGET (RCC) study, bleeding was reported in 15.3% of patients in the sorafenib-treated group and 8.2% of patients receiving placebo. The incidence of Grade 3 and 4 bleeding was 2% and 0%, respectively, in sorafenib-treated patients, and 1.3% and 0.2%, respectively, in those receiving placebo. There was one fatal hemorrhage in each treatment group in the TARGET (RCC) study. In the DECISION (DTC) study, bleeding was reported in 17.4% of sorafenib-treated patients and 9.6% of those receiving placebo; however, the incidence of Grade 3 bleeding was similar (1% and 1.4%) in sorafenib-treated patients and in those receiving placebo.
If any bleeding necessitates medical intervention, consider permanent discontinuation of sorafenib tablets [see Dosage and Administration (2.2)]. Due to the potential risk of bleeding, treat tracheal, bronchial, and esophageal infiltration with local therapy prior to administering sorafenib tablets in patients with DTC.
5.3 Hypertension
In the SHARP (HCC) study, hypertension was reported in 9.4% of sorafenib- treated patients and 4.3% of patients receiving placebo. In the TARGET (RCC) study, hypertension was reported in 16.9% of sorafenib-treated patients and 1.8% of patients receiving placebo. In the DECISION (DTC) study, hypertension was reported in 40.6% of sorafenib-treated patients and 12.4% of patients receiving placebo. Hypertension was usually mild to moderate, occurred early in the course of treatment, and was managed with standard antihypertensive therapy. Permanent discontinuation due to hypertension occurred in 1 of 297 sorafenib-treated patients in the SHARP (HCC) study, 1 of 451 sorafenib- treated patients in the TARGET (RCC) study, and 1 of 207 sorafenib-treated patients in the DECISION (DTC) study.
Monitor blood pressure weekly during the first 6 weeks of sorafenib tablets. Thereafter, monitor blood pressure and treat hypertension, if required, in accordance with standard medical practice. In cases of severe or persistent hypertension despite institution of antihypertensive therapy, consider temporary or permanent discontinuation of sorafenib tablets [see Dosage and Administration (2.2)].
5.4 Dermatologic Toxicities
Hand-foot skin reaction and rash represent the most common adverse reactions attributed to sorafenib tablets. Rash and hand-foot skin reaction are usually Grade 1 and 2 and generally appear during the first six weeks of treatment with sorafenib tablets. Permanent discontinuation of therapy due to hand-foot skin reaction occurred in 4 (1.3%) of 297 sorafenib-treated patients with HCC, 3 (0.7%) of 451 sorafenib-treated patients with RCC, and 11 (5.3%) of 207 sorafenib-treated patients with DTC.
Management of dermatologic toxicities may include topical therapies for symptomatic relief, temporary treatment interruption and/or dose reduction of sorafenib tablets, or in severe or persistent cases, permanent discontinuation of sorafenib tablets [see Dosage and Administration (2.2)].
There have been reports of severe dermatologic toxicities, including Stevens- Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). These cases may be life-threatening. Discontinue sorafenib tablets if SJS or TEN are suspected.
5.5 Gastrointestinal Perforation
Gastrointestinal perforation has been reported in less than 1% of patients taking sorafenib tablets. In some cases this was not associated with apparent intra-abdominal tumor. In the event of a gastrointestinal perforation, permanently discontinue sorafenib tablets.
5.6 Increased Risk of Bleeding with Concomitant Use of Warfarin
Infrequent bleeding or elevations in the International Normalized Ratio (INR) have been reported in some patients taking warfarin while on sorafenib tablets. Monitor patients taking concomitant warfarin regularly for changes in prothrombin time (PT), INR or clinical bleeding episodes.
5.7 Risk of Impaired Wound Healing
Impaired wound healing can occur in patients who receive drugs that inhibit the VEGF signaling pathway. Therefore, sorafenib tablets has the potential to adversely affect wound healing.
Withhold sorafenib tablets for at least 10 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of sorafenib tablets after resolution of wound healing complications has not been established.
5.8 Increased Mortality Observed with Sorafenib Administered in Combination
with Carboplatin/Paclitaxel and Gemcitabine/Cisplatin in Squamous Cell Lung Cancer
In a subset analysis of two randomized controlled trials in chemo-naive patients with Stage IIIB to IV non-small cell lung cancer, patients with squamous cell carcinoma experienced higher mortality with the addition of sorafenib compared to those treated with carboplatin/paclitaxel alone (HR 1.81; 95% CI 1.19, 2.74) and gemcitabine/cisplatin alone (HR 1.22; 95% CI 0.82, 1.80). The use of sorafenib tablets in combination with carboplatin/paclitaxel is contraindicated in patients with squamous cell lung cancer. Sorafenib tablets in combination with gemcitabine/cisplatin is not recommended in patients with squamous cell lung cancer. The safety and effectiveness of sorafenib has not been established in patients with non-small cell lung cancer.
5.9 QT Interval Prolongation
Sorafenib can prolong the QT/QTc interval. QT/QTc interval prolongation increases the risk for ventricular arrhythmias.
Avoid sorafenib tablets in patients with congenital long QT syndrome. Monitor electrolytes and electrocardiograms in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics. Correct electrolyte abnormalities (magnesium, potassium, calcium). Interrupt sorafenib tablets if QTc interval is greater than 500 milliseconds or for an increase from baseline of 60 milliseconds or greater [see Clinical Pharmacology (12.2)].
5.10 Drug-Induced Liver Injury
Sorafenib-induced hepatitis is characterized by a hepatocellular pattern of liver damage with significant increases of transaminases which may result in hepatic failure and death. Increases in bilirubin and INR may also occur. The incidence of severe drug-induced liver injury, defined as elevated transaminase levels above 20 times the upper limit of normal or transaminase elevations with significant clinical sequelae (for example, elevated INR, ascites, fatal, or transplantation), was two of 3,357 patients (0.06%) in a global monotherapy database.
Monitor liver function tests regularly. In case of significantly increased transaminases without alternative explanation, such as viral hepatitis or progressing underlying malignancy, discontinue sorafenib tablets [see Dosage and Administration (2.2)].
5.11 Embryo-Fetal Toxicity
Based on its mechanism of action and findings in animals, sorafenib may cause fetal harm when administered to a pregnant woman. Sorafenib caused embryo- fetal toxicities in animals at maternal exposures that were significantly lower than the human exposures at the recommended dose of 400 mg twice daily. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of sorafenib tablets. Advise male patients with female partners of reproductive potential and pregnant partners to use effective contraception during treatment and for 3 months following the last dose of sorafenib tablets [see Use in Specific Populations (8.1, 8.3)].
5.12 Impairment of Thyroid Stimulating Hormone Suppression in
Differentiated Thyroid Carcinoma
Sorafenib impairs exogenous thyroid suppression. In the DECISION (DTC) study, 99% of patients had a baseline thyroid stimulating hormone (TSH) level less than 0.5 mU/L. Elevation of TSH level above 0.5 mU/L was observed in 41% of sorafenib-treated patients as compared with 16% of those receiving placebo patients. For patients with impaired TSH suppression while receiving sorafenib tablets, the median maximal TSH was 1.6 mU/L and 25% had TSH levels greater than 4.4 mU/L.
Monitor TSH levels monthly and adjust thyroid replacement medication as needed in patients with DTC.
• Cardiovascular Events: Consider temporary or permanent discontinuation of sorafenib tablets. (2.2, 5.1)
• Hemorrhage: Discontinue sorafenib tablets if needed. (5.2)
• Hypertension: Monitor blood pressure weekly during the first 6 weeks and periodically thereafter. Consider temporary or permanent discontinuation for severe or persistent hypertension despite antihypertensive therapy. (5.3)
• Dermatologic Toxicities: Interrupt and/or decrease dose. Discontinue for severe or persistent reactions, or if Stevens-Johnson syndrome and toxic epidermal necrolysis is suspected. (5.4)
• Gastrointestinal Perforation: Discontinue sorafenib tablets. (5.5)
• Risk of Impaired Wound Healing: Withhold sorfenib tablets for at least 10 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of sorafenib tablets after resolution of wound healing complications has not been established. (5.7)
• QT Prolongation: Monitor electrocardiograms and electrolytes in patients at increased risk for ventricular arrhythmias. Correct electrolytes. Interrupt if QTc greater than 500 msec or increases greater than 60 msec from baseline. (2.2, 5.9, 12.2)
• Drug-Induced Liver Injury: Monitor liver function tests regularly; discontinue for unexplained transaminase elevations. (5.10)
• Embryo-Fetal Toxicity: Sorafenib may cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception. (5.11, 8.1, 8.3)
• Impairment of Thyroid Stimulating Hormone Suppression (TSH) in DTC: Monitor TSH monthly and adjust thyroid replacement therapy in patients with thyroid cancer. (5.12)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Sorafenib
Strong CYP3A4 Inducers
The concomitant use of sorafenib with rifampin, a strong CYP3A4 inducer decreased the mean AUC of sorafenib, which may decrease the antitumor activity [see Clinical Pharmacology (12.3)]. Avoid concomitant use of sorafenib with strong CYP3A4 inducers, when possible, because these drugs can decrease the systemic exposure to sorafenib.
Neomycin
The concomitant use of sorafenib with neomycin decreased the mean AUC of sorafenib, which may decrease the antitumor activity. Avoid concomitant use of sorafenib with neomycin. The effects of other antibiotics on the pharmacokinetics of sorafenib have not been studied [see Clinical Pharmacology (12.3)].
7.2 Concomitant Use of Warfarin
The concomitant use of sorafenib and warfarin may increase the risk of bleeding or increased the INR. Monitor INR and for clinical bleeding episodes in patients taking warfarin while receiving sorafenib tablets [see Warnings and Precautions (5.6)].
7.3 Drugs That Prolong the QT Interval
Sorafenib is associated with QTc interval prolongation. Avoid coadministration of sorafenib tablets with medicinal products with a known potential to prolong QT/QTc interval [see Warnings and Precautions (5.9), Clinical Pharmacology (12.2 )].
• Strong CYP3A Inducers: Avoid strong CYP3A4 inducers. (7.1)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with sorafenib. Sorafenib was clastogenic when tested in an in vitro mammalian cell assay (Chinese hamster ovary) in the presence of metabolic activation. Sorafenib was not mutagenic in the in vitro Ames bacterial cell assay or clastogenic in an in vivo mouse micronucleus assay. One intermediate in the manufacturing process, which is also present in the final drug substance (<0.15%), was positive for mutagenesis in an in vitro bacterial cell assay (Ames test) when tested independently.
No specific studies with sorafenib have been conducted in animals to evaluate the effect on fertility. However, results from the repeat-dose toxicity studies suggest there is a potential for sorafenib to impair reproductive function and fertility. Multiple adverse effects were observed in male and female reproductive organs, with the rat being more susceptible than mice or dogs. Typical changes in rats consisted of testicular atrophy or degeneration, degeneration of epididymis, prostate, and seminal vesicles, central necrosis of the corpora lutea and arrested follicular development. Sorafenib-related effects on the reproductive organs of rats were manifested at daily oral doses ≥5 mg/kg (30 mg/m2). This dose results in an exposure (AUC) that is approximately 0.5 times the AUC in patients at the recommended human dose. Dogs showed tubular degeneration in the testes at 30 mg/kg/day (600 mg/m2/day). This dose results in an exposure that is approximately 0.3 times the AUC at the recommended human dose. Oligospermia was observed in dogs at 60 mg/kg/day (1,200 mg/m2/day) of sorafenib.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hepatocellular Carcinoma
The SHARP (HCC) study (NCT00105443) was an international, multicenter, randomized, double blind, placebo-controlled trial in patients with unresectable hepatocellular carcinoma. Overall survival was the primary endpoint. A total of 602 patients were randomized; 299 to sorafenib 400 mg twice daily and 303 to matching placebo. All 602 randomized subjects were included in the ITT population for the efficacy analyses.
Demographics and baseline disease characteristics were similar between the sorafenib and placebo arms with regard to age, gender, race, performance status, etiology (including hepatitis B, hepatitis C and alcoholic liver disease), TNM stage (stage I: <1% vs. <1%; stage II: 10.4% vs. 8.3%; stage III: 37.8% vs. 43.6%; stage IV: 50.8% vs. 46.9%), absence of both macroscopic vascular invasion and extrahepatic tumor spread (30.1% vs. 30%), and Barcelona Clinic Liver Cancer stage (stage B: 18.1% vs. 16.8%; stage C: 81.6% vs. 83.2%; stage D: <1% vs. 0%). Liver impairment by Child-Pugh score was comparable between the sorafenib and placebo arms (Class A: 95% vs. 98%; B: 5% vs. 2%). Only one patient with Child-Pugh class C was entered. Prior treatments included surgical resection procedures (19.1% vs. 20.5%), locoregional therapies (including radiofrequency ablation, percutaneous ethanol injection and transarterial chemoembolization; 38.8% vs. 40.6%), radiotherapy (4.3% vs. 5%) and systemic therapy (3% vs. 5%).
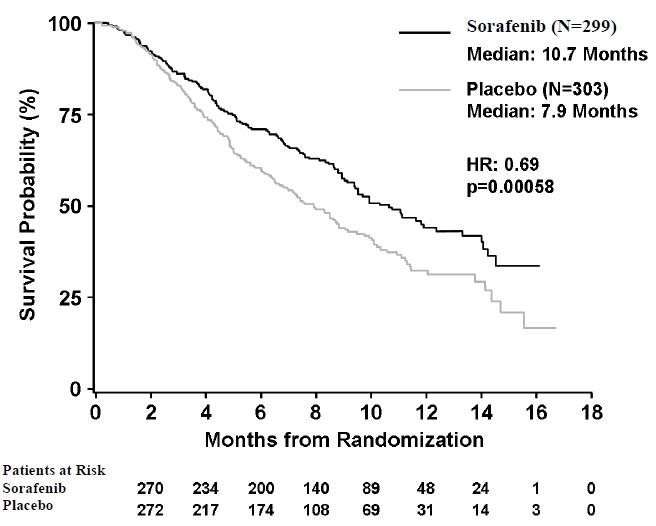
The trial was stopped for efficacy following a pre-specified second interim analysis for survival showing a statistically significant advantage for sorafenib over placebo for overall survival (HR: 0.69, p=0.00058) (see Table 10 and Figure 1). This advantage was consistent across all subsets analyzed.
Final analysis of time to tumor progression (TTP) based on data from an earlier time point (by independent radiologic review) also was significantly longer in the sorafenib arm (HR: 0.58, p=0.000007) (see Table 10).
Table 10: Efficacy Results from SHARP (HCC)
|
Efficacy Parameter |
Sorafenib**** |
Placebo**** |
|
Overall Survival | ||
|
Number of Events |
143 |
178 |
|
Median, months |
10.7 |
7.9 |
|
(95% CI) |
(9.4, 13.3) |
(6.8, 9.1) |
|
Hazard Ratio1 (95% CI) |
0.69 (0.55, 0.87) | |
|
P-value (log-rank test2) |
0.00058 | |
|
Time to Progression****3 | ||
|
Number of Events |
107 |
156 |
|
Median, months |
5.5 |
2.8 |
|
(95% CI) |
(4.1, 6.9) |
(2.7, 3.9) |
|
Hazard Ratio1 (95% CI) |
0.58 | |
|
P-value (log-rank test2) |
0.000007 |
CI=Confidence interval
1 Hazard ratio, sorafenib/placebo, stratified Cox model
2 Stratified log rank (for the interim analysis of survival, the stopping boundary one-sided alpha = 0.0077)
3 The time-to-progression (TTP) analysis, based on independent radiologic review, was based on data from an earlier time point than the survival analysis
Figure 1: Kaplan-Meier Curve of Overall Survival in SHARP (HCC) (Intent-to- Treat Population)
14.2 Renal Cell Carcinoma
The safety and efficacy of sorafenib in the treatment of advanced renal cell carcinoma (RCC) were studied in the following two randomized controlled clinical trials.
TARGET
TARGET (NCT00073307) was an international, multicenter, randomized, double blind, placebo-controlled trial in patients with advanced renal cell carcinoma who had received one prior systemic therapy. Primary study endpoints included overall survival and progression-free survival (PFS). Tumor response rate was a secondary endpoint. The PFS analysis included 769 patients, per protocol, stratified by MSKCC (Memorial Sloan Kettering Cancer Center) prognostic risk category (low or intermediate) and country and randomized to sorafenib 400 mg twice daily (N=384) or to placebo (N=385).
Table 11 summarizes the demographic and disease characteristics of the study population analyzed. Baseline demographics and disease characteristics were well balanced for both treatment groups. The median time from initial diagnosis of RCC to randomization was 1.6 and 1.9 years for the sorafenib and placebo arms, respectively.
Table 11: Demographic and Disease Characteristics – TARGET (RCC)
|
Characteristics |
Sorafenib**** |
Placebo****N=385 | ||
|
N |
(%) |
N |
(%) | |
|
Gender | ||||
|
Male |
267 |
(70) |
287 |
(75) |
|
Female |
116 |
(30) |
98 |
(25) |
|
Race | ||||
|
White |
276 |
(72) |
278 |
(73) |
|
Black/Asian/ Hispanic/Other |
11 |
(3) |
10 |
(2) |
|
Not reported1 |
97 |
(25) |
97 |
(25) |
|
Age group | ||||
|
< 65 years |
255 |
(67) |
280 |
(73) |
|
≥ 65 years |
127 |
(33) |
103 |
(27) |
|
ECOG performance status at baseline | ||||
|
0 |
184 |
(48) |
180 |
(47) |
|
1 |
191 |
(50) |
201 |
(52) |
|
2 |
6 |
(2) |
1 |
(<1) |
|
Not reported |
3 |
(<1) |
3 |
(<1) |
|
MSKCC prognostic risk category | ||||
|
Low |
200 |
(52) |
194 |
(50) |
|
Intermediate |
184 |
(48) |
191 |
(50) |
|
Prior IL-2 and/or interferon | ||||
|
Yes |
319 |
(83) |
313 |
(81) |
|
No |
65 |
(17) |
72 |
(19) |
1 Race was not collected from the 186 patients enrolled in France due to local regulations. In 8 other patients, race was not available at the time of analysis.
Progression-free survival, defined as the time from randomization to progression or death from any cause, whichever occurred earlier, was evaluated by blinded independent radiological review using RECIST criteria. Figure 2 depicts Kaplan-Meier curves for PFS. The PFS analysis was based on a two-sided Log-Rank test stratified by MSKCC prognostic risk category and country.
Figure 2: Kaplan-Meier Curves for Progression-free Survival – TARGET (RCC)
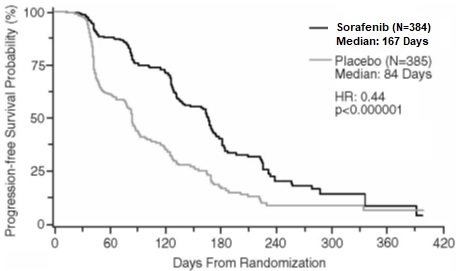
NOTE: HR is from Cox regression model with the following covariates: MSKCC prognostic risk category and country. P-value is from two-sided Log-Rank test stratified by MSKCC prognostic risk category and country.
The median PFS for patients randomized to sorafenib was 167 days compared to 84 days for patients randomized to placebo. The estimated hazard ratio (immediate risk of progression or death with sorafenib compared to placebo) was 0.44 (95% CI: 0.35, 0.55).
A series of patient subsets were examined in exploratory univariate analyses of PFS. The subsets included age above or below 65 years, ECOG PS 0 or 1, MSKCC prognostic risk category, whether the prior therapy was for progressive metastatic disease or for an earlier disease setting and time from diagnosis of less than or greater than 1.5 years. The effect of sorafenib on PFS was consistent across these subsets, including patients with no prior IL-2 or interferon therapy (N=137; 65 patients receiving sorafenib and 72 placebo), for whom the median PFS was 172 days in the sorafenib arm compared to 85 days in the placebo arm.
Tumor response was determined by independent radiologic review according to RECIST criteria. Overall, of 672 patients who were evaluable for response, 7 (2%) patients in the sorafenib and no (0%) patients in the placebo arms had a confirmed partial response. Thus the gain in PFS primarily reflects the stable disease population.
At the time of a planned interim survival analysis, based on 220 deaths, overall survival was longer for those randomized to sorafenib compared with placebo with a hazard ratio of 0.72. This analysis did not meet the prespecified criteria for statistical significance. Additional analyses are planned as the survival data mature.
BAY43-9006
BAY43-9006 (NCT00101413) was a randomized discontinuation trial in patients with metastatic malignancies, including RCC. The primary endpoint was the percentage of randomized patients remaining progression-free at 24 weeks. All patients received sorafenib for the first 12 weeks. Radiologic assessment was repeated at week 12. Patients with <25% change in bi-dimensional tumor measurements from baseline were randomized to sorafenib or placebo for a further 12 weeks. Patients who were randomized to placebo were permitted to cross over to open-label sorafenib upon progression. Patients with tumor shrinkage ≥25% continued sorafenib, whereas patients with tumor growth ≥25% discontinued treatment.
A total of 202 patients with advanced RCC were enrolled into BAY43-9006, including patients who had received no prior therapy and patients with tumor histology other than clear cell carcinoma. After the initial 12 weeks of sorafenib, 79 patients with RCC continued on open-label sorafenib, and 65 patients were randomized to sorafenib or placebo. After an additional 12 weeks, at week 24, for the 65 randomized patients, the progression-free rate was significantly higher in patients randomized to sorafenib (16/32, 50%) than in patients randomized to placebo (6/33, 18%) (p=0.0077). Progression-free survival was significantly longer in the sorafenib arm (163 days) than in the those randomized to placebo (41 days) (p=0.0001, HR=0.29).
14.3 Differentiated Thyroid Carcinoma
The safety and effectiveness of sorafenib was evaluated in a multicenter, randomized (1:1), double-blind, placebo-controlled trial (DECISION; NCT00984282) conducted in 417 patients with locally recurrent or metastatic, progressive differentiated thyroid carcinoma (DTC) refractory to radioactive iodine (RAI) treatment. Randomization was stratified by age (< 60 years versus ≥ 60 years) and geographical region (North America, Europe, and Asia). All 417 subjects were included in the ITT population for the efficacy analyses.
All patients were required to have actively progressing disease defined as progression within 14 months of enrollment. RAI-refractory disease was defined based on four criteria that were not mutually exclusive. All RAI treatments and diagnostic scans were to be performed under conditions of a low iodine diet and adequate TSH stimulation. Following are the RAI-refractory criteria and the proportion of patients in the study that met each one: a target lesion with no iodine uptake on RAI scan (68%); tumors with iodine uptake and progression after RAI treatment within 16 months of enrollment (12%); tumors with iodine uptake and multiple RAI treatments with the last treatment greater than 16 months prior to enrollment, and disease progression after each of two RAI treatments administered within 16 months of each other (7%); cumulative RAI dose ≥ 600 mCi administered (34%). The major efficacy outcome measure was progression-free survival (PFS) as determined by a blinded, independent radiological review using a modified Response Evaluation Criteria in Solid Tumors v. 1.0 (RECIST). RECIST was modified by inclusion of clinical progression of bone lesions based on the need for external beam radiation (4.4% of progression events). Additional efficacy outcomes measures included overall survival (OS), tumor response rate, and duration of response.
Patients were randomized to receive sorafenib 400 mg twice daily (n=207) or placebo (n=210). Of the 417 patients randomized, 48% were male, the median age was 63 years, 61% were 60 years or older, 60% were white, 62% had an ECOG performance status of 0, and 99% had undergone thyroidectomy. The histological diagnoses were papillary carcinoma in 57%, follicular carcinoma (including Hürthle cell) in 25%, and poorly differentiated carcinoma in 10%, and other in 8% of the study population. Metastases were present in 96% of the patients: lungs in 86%, lymph nodes in 51%, and bone in 27%. The median cumulative RAI activity administered prior to study entry was 400 mCi.
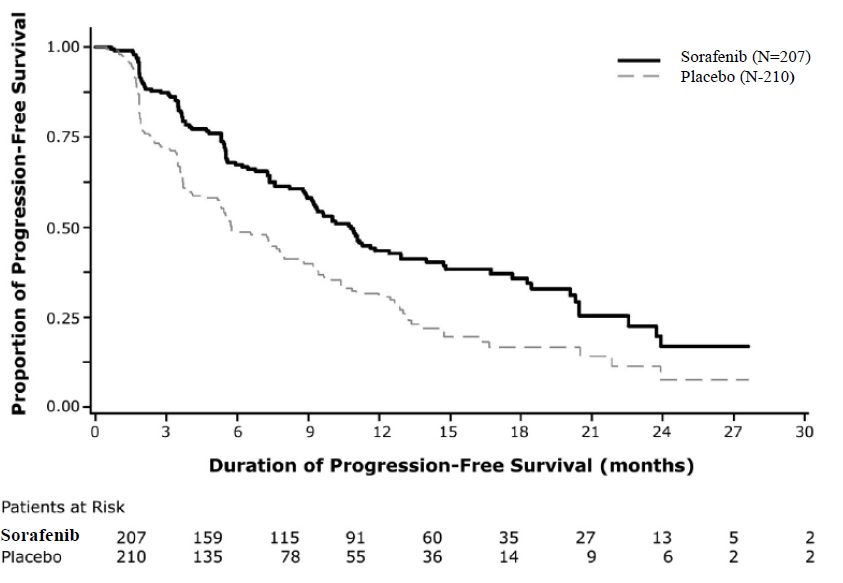
A statistically significant prolongation of PFS was demonstrated for sorafenib-treated patients compared to those receiving placebo (Figure 3); no statistically significant difference was seen in the final overall survival (OS) analysis (Table 12). Crossover to open label sorafenib occurred in 161 (77%) patients randomized to placebo after investigator-determined disease progression.
Table 12: Efficacy Results from DECISION in Differentiated Thyroid Carcinoma
|
Sorafenib**** |
Placebo**** | |
|
Progression-free Survival****1 | ||
|
Number of Deaths or Progression |
113 (55%) |
136 (65%) |
|
Median PFS in Months (95% CI) |
10.8 (9.1, 12.9) |
5.8 ( 5.3, 7.8) |
|
Hazard Ratio (95% CI) |
0.59 (0.46, 0.76) | |
|
P-value 2 |
<0.001 | |
|
Overall Survival****3 | ||
|
Number of Deaths |
103 (49.8%) |
109 (51.9%) |
|
Median OS in Months (95% CI) |
42.8 (34.6, 52.6) |
39.4 (32.7, 51.4) |
|
Hazard Ratio (95% CI) |
0.92 (0.71, 1.21) | |
|
P-value2 |
0.570 | |
|
Objective Response | ||
|
Number of Objective Responders 4 |
24 (12%) |
1 (0.5%) |
|
(95% CI) |
(7.6%, 16.8%) |
(0.01%, 2.7%) |
|
Median Duration of Response in Months (95% CI) |
10.2 (7.4, 16.6) |
NE |
1 Independent radiological review
2 Two-sided log-rank test stratified by age (< 60 years, ≥ 60 years) and geographic region (North America, Europe, Asia)
3 Conducted after 212 events, which occurred 36 months after the primary PFS analysis.
4 All objective responses Se partial responses NR = Not Reached, CI = Confidence interval, NE = Not Estimable
Figure 3: Kaplan-Meier Curve of Progression-Free Survival in DECISION (DTC)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read FDA-approved patient labeling (Patient Information).
Cardiovascular Events
Discuss with patients that cardiac ischemia and/or infarction and congestive heart failure, have been reported during sorafenib treatment, and that they should immediately report any episodes of chest pain or other symptoms of cardiac ischemia or congestive heart failure [see Warnings and Precautions (5.1)].
Bleeding
Inform patients that sorafenib tablets can increase the risk of bleeding and that they should promptly report any episodes of bleeding [see Warnings and Precautions (5.2)].
Inform patients that bleeding or elevations in the International Normalized Ratio (INR) have been reported in some patients taking warfarin while on sorafenib tablets and that their INR should be monitored regularly [see Warnings and Precautions (5.6)].
Hypertension
Inform patients that hypertension can develop during sorafenib tablets treatment, especially during the first six weeks of therapy, and that blood pressure should be monitored regularly during treatment [see Warnings and Precautions (5.3)].
Skin Reactions
Advise patients of the possible occurrence of hand-foot skin reaction and rash during sorafenib tablets treatment and appropriate counter measures [see Warnings and Precautions (5.4)].
Gastrointestinal Perforation
Advise patients that cases of gastrointestinal perforation have been reported in patients taking sorafenib tablets [see Warnings and Precautions (5.5)].
Risk of Impaired Wound Healing
Advise patients that sorafenib tablets may impair wound healing. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.7)].
QT Interval Prolongation
Inform patients with a history of prolonged QT interval that sorafenib tablets can worsen the condition [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.2)].
Drug-Induced Liver Injury
Inform patients that sorafenib tablets can cause hepatitis which may result in hepatic failure and death. Advise patients that liver function tests should be monitored regularly during treatment and to report signs and symptoms of hepatitis [see Warnings and Precautions (5.10)].
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of pregnancy [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with sorafenib tablets and for 6 months after the last dose. Advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment with sorafenib tablets and for 3 months after receiving the last dose of sorafenib tablets [see Warnings and Precautions (5.11), Use in Specific Populations (8.1,8.3)].
Lactation
Advise patients not to breastfeed while taking sorafenib tablets and for 2 weeks after receiving the last dose of sorafenib tablets [see Use in Specific Populations (8.2)].
Missed Doses
Instruct patients that if a dose of sorafenib tablets is missed, the next dose should be taken at the regularly scheduled time, and not double the dose. Instruct patients to contact their healthcare provider immediately if they take too much sorafenib tablets.
Rx only
Distributor:
Dr. Reddy’s Laboratories Inc.,
Princeton, NJ 08540
Made in India
Revised: 11/2022
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
**Sorafenib (soe raf' e nib)**Tablets, USP
oral
|
What is sorafenib tablet? |
|
Do not take sorafenib tablets if you: |
|
Before taking sorafenib tablets, tell your healthcare provider about all of
your medical conditions including if you: For maleswith female partners who are able to become pregnant: |
|
How should I take sorafenib tablets? |
|
What are the possible side effects of sorafenib tablets?**** The most common side effects of sorafenib tablets include: ******•**diarrhea (frequent or loose bowel movements) These are not all of the possible side effects of sorafenib tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store sorafenib tablets? |
|
General information about the safe and effective use of****sorafenib
tablets |
|
What are the ingredients in sorafenib tablets?**** Inactive Ingredients: croscarmellose sodium, hypromellose, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium lauryl sulphate, talc and titanium dioxide. For more information, call Dr. Reddy’s Laboratories Inc., at 1-888-375-3784. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
To reorder additional Patient Information Sheets, contact Dr. Reddy’s Customer Service at 1-866-733-3952.
Rx only
Distributor:
Dr. Reddy’s Laboratories Inc.,
Princeton, NJ 08540
Made in India
Revised: 11/2022
Patient Information Sheet also available at: www.drreddys.com/pil/sorafenibtabs.pdf
