Mucus Relief
CVS Guaifenesin Extended-Release Tablets 1200 mg
2cfbb804-de2b-5b2f-e063-6394a90a0824
HUMAN OTC DRUG LABEL
May 23, 2025
CVS Pharmacy
DUNS: 062312574
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Guaifenesin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
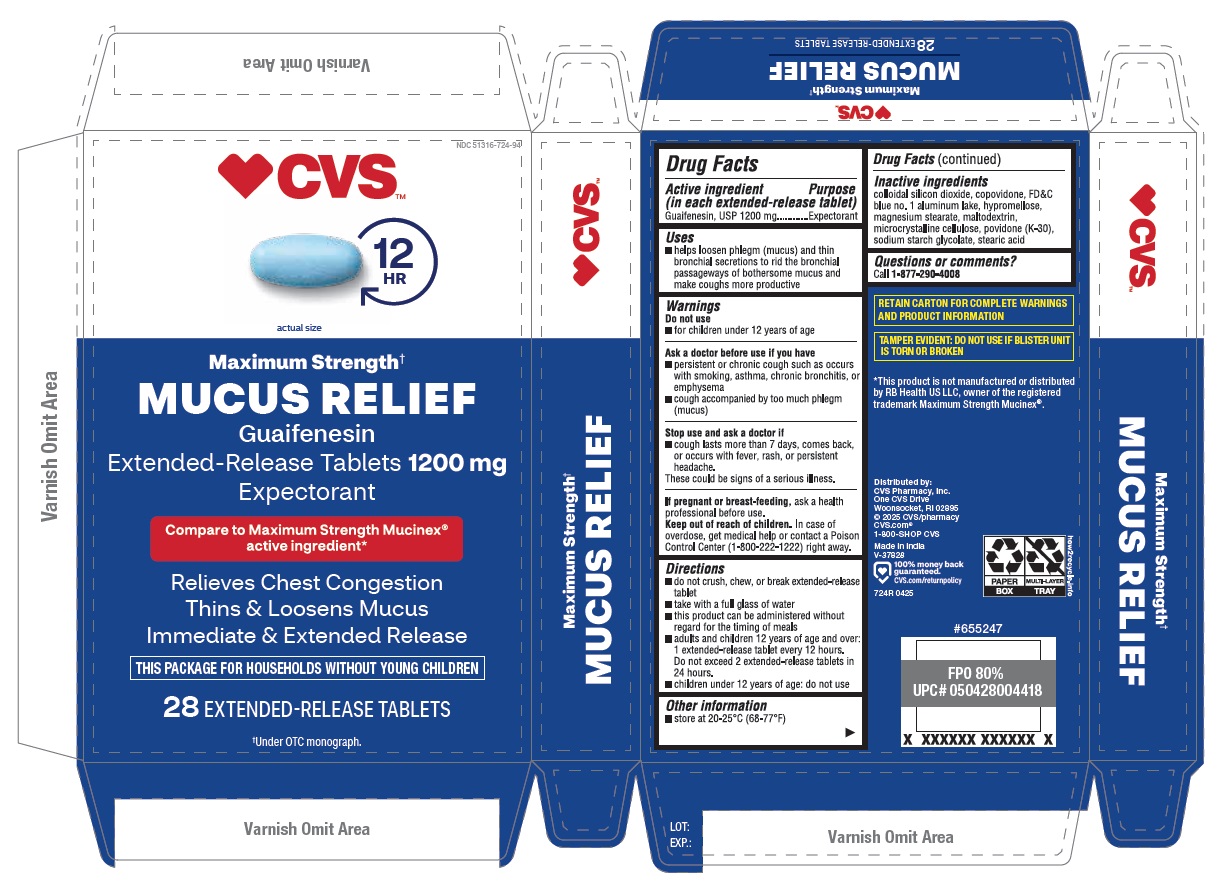
INDICATIONS & USAGE SECTION
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
OTC - STOP USE SECTION
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
SPL UNCLASSIFIED SECTION
Drug Facts
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each extended-release tablet)
Guaifenesin, USP 1200 mg
OTC - PURPOSE SECTION
Purpose
Expectorant
WARNINGS SECTION
Warnings
OTC - DO NOT USE SECTION
Do not use
- for children under 12 years of age
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
DOSAGE & ADMINISTRATION SECTION
Directions
- do not crush, chew, or break extended-release tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 extended-release tablet every 12 hours. Do not exceed 2 extended-release tablets in 24 hours.
- children under 12 years of age: do not use
OTHER SAFETY INFORMATION
Other information
- store at 20-25°C (68-77°F)
INACTIVE INGREDIENT SECTION
Inactive ingredients colloidal silicon dioxide, copovidone, FD&C blue no. 1 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone (K-30), sodium starch glycolate, stearic acid.
OTC - QUESTIONS SECTION
Questions or comments? Call1-877-290-4008
