Naproxen
These highlights do not include all the information needed to use NAPROXEN TABLETS safely and effectively. See full prescribing information for NAPROXEN TABLETS. NAPROXEN tablets, for oral use Initial U.S. Approval: 1976
49a00870-f580-4eb9-a95d-48fa956f147f
HUMAN PRESCRIPTION DRUG LABEL
Apr 1, 2024
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Naproxen
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
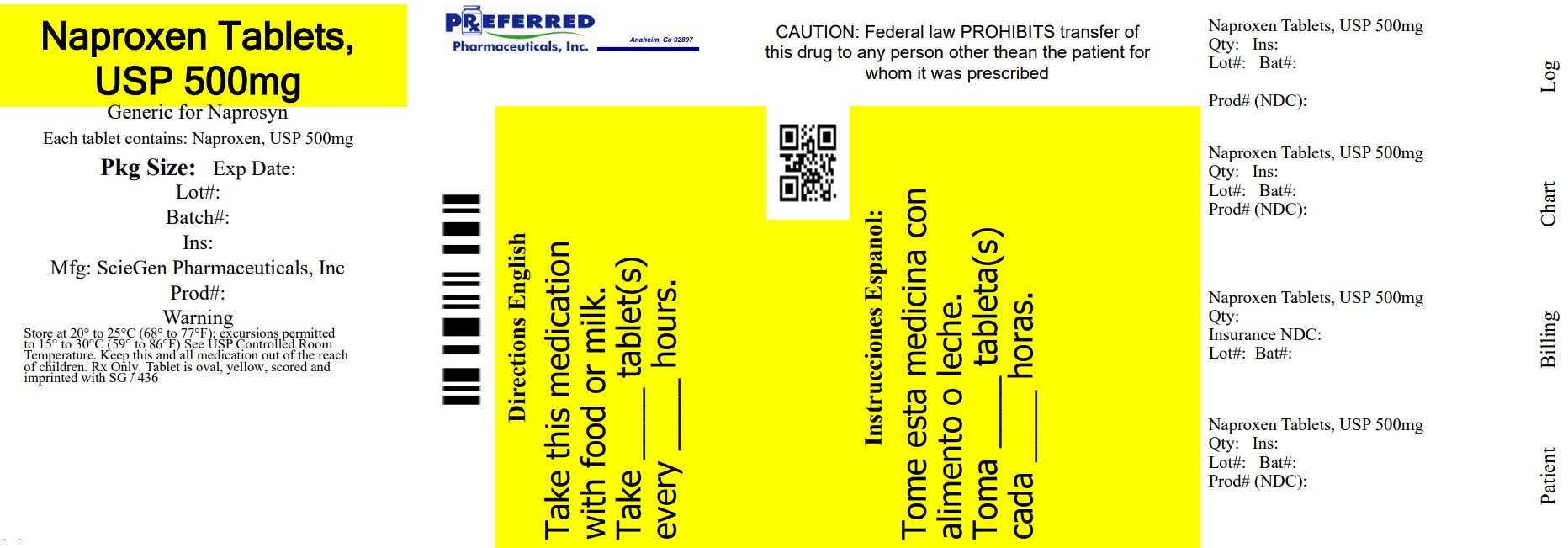
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 68788-8602
Naproxen Tablets, USP
500mg
Repackaged By: Preferred Pharmaceuticals Inc.
Boxed Warning section
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Naproxen Tablets are indicated for:
the relief of the signs and symptoms of:
•
rheumatoid arthritis
•
osteoarthritis
•
ankylosing spondylitis
•
polyarticular juvenile idiopathic arthritis
•
tendonitis
•
bursitis
•
acute gout
the management of:
•
pain
•
primary dysmenorrhea
Naproxen tablets are non-steroidal anti-inflammatory drugs indicated for:
the relief of the signs and symptoms of: (1)
•
rheumatoid arthritis
•
osteoarthritis
•
ankylosing spondylitis
•
polyarticular juvenile idiopathic arthritis
•
tendonitis
•
bursitis
•
acute gout
the management of: (1)
•
pain
•
primary dysmenorrhea
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Naproxen tablets are contraindicated in the following patients:
•
Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to naproxen or any components of the drug product [ see Warnings and Precautions (5.7, 5.9)]
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [ see Warnings and Precautions (5.7, 5.8)]
•
In the setting of coronary artery bypass graft (CABG) surgery [ see Warnings and Precautions (5.1)]
•
Known hypersensitivity to naproxen or any components of the drug product (4)
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4)
•
In the setting of CABG surgery (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
•
Cardiovascular Thrombotic Events [ see Warnings and Precautions (5.1)]
•
GI Bleeding, Ulceration, and Perforation [ see Warnings and Precautions (5.2)]
•
Hepatotoxicity [ see Warnings and Precautions (5.3)]
•
Hypertension [ see Warnings and Precautions (5.4)]
•
Heart Failure and Edema [ see Warnings and Precautions (5.5)]
•
Renal Toxicity and Hyperkalemia [ see Warnings and Precautions (5.6)]
•
Anaphylactic Reactions [ see Warnings and Precautions (5.7)]
•
Serious Skin Reactions [ see Warnings and Precautions (5.9)]
•
Hematologic Toxicity [ see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported in controlled clinical trials in 960 patients treated for rheumatoid arthritis or osteoarthritis are listed below. In general, reactions in patients treated chronically were reported 2 to 10 times more frequently than they were in short-term studies in the 962 patients treated for mild to moderate pain or for dysmenorrhea. The most frequent complaints reported related to the gastrointestinal tract.
A clinical study found gastrointestinal reactions to be more frequent and more severe in rheumatoid arthritis patients taking daily doses of 1,500 mg naproxen compared to those taking 750 mg naproxen.
In controlled clinical trials with about 80 pediatric patients and in well- monitored, open-label studies with about 400 pediatric patients with polyarticular juvenile idiopathic arthritis treated with naproxen, the incidence of rash and prolonged bleeding times were greater, the incidence of gastrointestinal and central nervous system reactions were about the same, and the incidence of other reactions were lower in pediatric patients than in adults.
In patients taking naproxen in clinical trials, the most frequently reported adverse experiences in approximately 1% to 10% of patients were:
Gastrointestinal (GI) Experiences, including:heartburn *, abdominal pain *, nausea *, constipation *, diarrhea, dyspepsia, stomatitis
Central Nervous System:headache *, dizziness *, drowsiness *, lightheadedness, vertigo
Dermatologic:pruritus (itching) *, skin eruptions *, ecchymoses *, sweating, purpura
Special Senses:tinnitus *, visual disturbances, hearing disturbances
Cardiovascular:edema *, palpitations
General:dyspnea *, thirst
*Incidence of reported reaction between 3% and 9%. Those reactions occurring in less than 3% of the patients are unmarked.
In patients taking NSAIDs, the following adverse experiences have also been reported in approximately 1% to 10% of patients.
Gastrointestinal (GI) Experiences, including:flatulence, gross bleeding/perforation, GI ulcers (gastric/duodenal), vomiting
General:abnormal renal function, anemia, elevated liver enzymes, increased bleeding time, rashes
The following are additional adverse experiences reported in <1% of patients taking naproxen during clinical trials.
Gastrointestinal:pancreatitis, vomiting
Hepatobiliary:jaundice
Hemic and Lymphatic:melena, thrombocytopenia, agranulocytosis
Nervous System:inability to concentrate
Dermatologic:skin rashes
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of naproxen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following are additional adverse experiences reported in <1% of patients taking naproxen during clinical trials and through postmarketing reports. Those adverse reactions observed through postmarketing reports are italicized.
Body as a Whole:anaphylactoid reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever)
Cardiovascular:congestive heart failure, vasculitis, hypertension, pulmonary edema
Gastrointestinal:inflammation, bleeding (sometimes fatal, particularly in the elderly), ulceration, perforation and obstruction of the upper or lower gastrointestinal tract. Esophagitis, stomatitis, hematemesis, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn’s disease).
Hepatobiliary:abnormal liver function tests, hepatitis (some cases have been fatal)
Hemic and Lymphatic:eosinophilia, leucopenia, granulocytopenia, hemolytic anemia, aplastic anemia
Metabolic and Nutritional:hyperglycemia, hypoglycemia
Nervous System:depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions
Respiratory:eosinophilic pneumonitis, asthma
Dermatologic:alopecia, urticaria, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens- Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored.
Special Senses:hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema
Urogenital:glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine
Reproduction (female):infertility
In patients taking NSAIDs, the following adverse experiences have also been reported in <1% of patients.
Body as a Whole:fever, infection, sepsis, anaphylactic reactions, appetite changes, death
Cardiovascular:hypertension, tachycardia, syncope, arrhythmia, hypotension, myocardial infarction
Gastrointestinal:dry mouth, esophagitis, gastric/peptic ulcers, gastritis, glossitis, eructation
Hepatobiliary:hepatitis, liver failure
Hemic and Lymphatic:rectal bleeding, lymphadenopathy, pancytopenia
Metabolic and Nutritional:weight changes
Nervous System:anxiety, asthenia, confusion, nervousness, paresthesia, somnolence, tremors, convulsions, coma, hallucinations
Respiratory:asthma, respiratory depression, pneumonia
Dermatologic:exfoliative dermatitis
Special Senses:blurred vision, conjunctivitis
Urogenital:cystitis, dysuria, oliguria/polyuria, proteinuria
Most common adverse reactions to naproxen were dyspepsia, abdominal pain, nausea, headache, rash, ecchymosis, and edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ScieGen Pharmaceuticals, Inc. at 1-855-724-3436 or FDA at 1-800-FDA-1088 or****Error! Hyperlink reference not valid.
DESCRIPTION SECTION
11 DESCRIPTION
Naproxen tablets, USP are nonsteroidal anti-inflammatory drugs and available as follows: Naproxen tablets, USP are available as light yellow round shaped tablets containing 250 mg naproxen, light yellow capsule shaped tablets containing 375 mg naproxen, and light yellow oblong shaped tablets containing 500 mg naproxen for oral administration.
Naproxen is a propionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs. The chemical name for naproxen is (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid. Naproxen has a molecular weight of 230.26 and a molecular formula of C 14H 14O 3. It has the following structural formula:
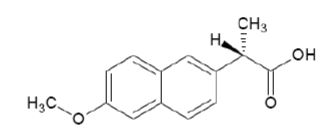
Naproxen is white or almost white crystalline powder. It is insoluble in water, soluble in chloroform, dehydrated ethanol and methanol. Sparingly soluble in ether. The octanol/water partition coefficient of Naproxen at pH < 2.18 is 3.18.
Each naproxen tablet, USP contains the following inactive ingredients: croscarmellose sodium, yellow iron oxide, povidone and magnesium stearate
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Warnings and Precautions ( 5.10, 5.11) |
04/2021 |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Naproxen Tablets USP, 250 mg are light yellow, round shaped tablets debossed with "SG" along with break-line on one side and"434" on the other side.
Naproxen Tablets USP, 375 mg are light yellow, capsule shaped tablets debossed with "SG" on one side and "435" on the other side.
Naproxen Tablets USP, 500 mg are light yellow, oblong shaped tablets debossed with "SG" along with break-line on one side and "436" on the other side.
Naproxen tablets: 250 mg, 375 mg and 500 mg (3)
OVERDOSAGE SECTION
10 OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [ see Warnings and Precautions ( 5.1, 5.2) ]. A few patients have experienced convulsions, but it is not clear whether or not these were drug-related. It is not known what dose of the drug would be life threatening. [see Warnings and Precautions ( 5.1, 5.2, 5.4, 5.6)].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Consider emesis and/or activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Naproxen has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of naproxen, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Naproxen is a potent inhibitor of prostaglandin synthesis in vitro. Naproxen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because naproxen is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.2 Pharmacodynamics
In a healthy volunteer study, 10 days of concomitant administration of naproxen 220 mg once-daily with low-dose immediate-release aspirin (81 mg) showed an interaction with the antiplatelet activity of aspirin as measured by % serum thromboxane B2 inhibition at 24 hours following the day 10 dose [98.7% (aspirin alone) vs 93.1% (naproxen and aspirin)]. The interaction was observed even following discontinuation of naproxen on day 11 (while aspirin dose was continued) but normalized by day 13. In the same study, the interaction was greater when naproxen was administered 30 minutes prior to aspirin [98.7% vs 87.7%] and minimal when aspirin was administered 30 minutes prior to naproxen [98.7% vs 95.4%].
Following administration of naproxen 220 mg twice-daily with low-dose immediate-release aspirin (first naproxen dose given 30 minutes prior to aspirin), the interaction was minimal at 24 h following day 10 dose [98.7% vs 95.7%]. However, the interaction was more prominent after discontinuation of naproxen (washout) on day 11 [98.7% vs 84.3%] and did not normalize completely by day 13 [98.5% vs 90.7%]. [ see Drug Interactions (7)].
12.3 Pharmacokinetics
Naproxen is rapidly and completely absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%. The elimination half-life of naproxen is unchanged across products ranging from 12 to 17 hours. Steady-state levels of naproxen are reached in 4 to 5 days, and the degree of naproxen accumulation is consistent with this half-life.
Absorption
After administration of naproxen tablets, peak plasma levels are attained in 2 to 4 hours.
Distribution
Naproxen has a volume of distribution of 0.16 L/kg. At therapeutic levels naproxen is greater than 99% albumin-bound. At doses of naproxen greater than 500 mg/day there is less than proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough C ss36.5, 49.2 and 56.4 mg/L with 500, 1,000 and 1,500 mg daily doses of naproxen, respectively). The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma [ see Use in Specific Populations (8.2)].
Elimination
Metabolism
Naproxen is extensively metabolized in the liver to 6-0-desmethyl naproxen, and both parent and metabolites do not induce metabolizing enzymes. Both naproxen and 6-0-desmethyl naproxen are further metabolized to their respective acylglucuronide conjugated metabolites.
Excretion
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (<1%), 6-0-desmethyl naproxen (<1%) or their conjugates (66% to 92%). The plasma half-life of the naproxen anion in humans ranges from 12 to 17 hours. The corresponding half-lives of both naproxen’s metabolites and conjugates are shorter than 12 hours, and their rates of excretion have been found to coincide closely with the rate of naproxen clearance from the plasma. Small amounts, 3% or less of the administered dose, are excreted in the feces. In patients with renal failure metabolites may accumulate [ see Warnings and Precautions (5.6)].
Specific Populations
Pediatric:
In pediatric patients aged 5 to 16 years with arthritis, plasma naproxen levels following a 5 mg/kg single dose of naproxen suspension [ see Dosage and Administration (2)] were found to be similar to those found in normal adults following a 500 mg dose. The terminal half-life appears to be similar in pediatric and adult patients. Pharmacokinetic studies of naproxen were not performed in pediatric patients younger than 5 years of age. Pharmacokinetic parameters appear to be similar following administration of naproxen suspension or tablets in pediatric patients.
Geriatric:
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly, although the unbound fraction is <1% of the total naproxen concentration. Unbound trough naproxen concentrations in elderly subjects have been reported to range from 0.12% to 0.19% of total naproxen concentration, compared with 0.05% to 0.075% in younger subjects.
Hepatic Impairment:
Naproxen pharmacokinetics has not been determined in subjects with hepatic insufficiency.
Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased.
Renal Impairment:
Naproxen pharmacokinetics has not been determined in subjects with renal insufficiency. Given that naproxen, its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen metabolites to accumulate in the presence of renal insufficiency. Elimination of naproxen is decreased in patients with severe renal impairment.
Drug Interaction Studies
Aspirin:When NSAIDs were administered with aspirin, the protein binding of
NSAIDs were reduced, although the clearance of free NSAID was not altered. The
clinical significance of this interaction is not known. See Table 1 for
clinically significant drug interactions of NSAIDs with aspirin [ see Drug Interactions (7)].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with naproxen tablets and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [ see Warnings and Precautions (5.1)].
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [ see Warnings and Precautions (5.2)].
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, instruct patients to stop naproxen tablets and seek immediate medical therapy [ see Warnings and Precautions (5.3)].
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [ see Warnings and Precautions (5.5)].
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur [ see Contraindications (4)and Warnings and Precautions (5.7)].
Serious Skin Reactions, including DRESS
Advise patients to stop taking Naproxen tablets immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible [ see Warnings and Precautions (5.9, 5.10)].
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including naproxen tablets, may be associated with a reversible delay in ovulation [ see Use in Specific Populations (8.3)]
Fetal Toxicity
Inform pregnant women to avoid use of naproxen tablets and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with naproxen tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [ see Warnings and Precautions (5.11)and Use in Specific Populations (8.1)].
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of Naproxen tablets with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [ see Warnings and Precautions (5.2)and Drug Interactions (7)]. Alert patients that NSAIDs may be present in “over the counter” medications for treatment of colds, fever, or insomnia.
Use of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with Naproxen tablets until they talk to their healthcare provider [ see Drug Interactions (7)].
Manufactured by:
ScieGen Pharmaceuticals Inc
Hauppauge, NY 11788 USA
Rev. 5/2021
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Naproxen Tablets USP, 500 mg are light yellow, oblong shaped tablets debossed with “S & G” on either side of functional score on one side and “436” on the other side.
Bottles of 14 NDC 68788-8602-7
Bottles of 15 NDC 68788-8602-5
Bottles of 20 NDC 68788-8602-2
Bottles of 30 NDC 68788-8602-3
Bottles of 40 NDC 68788-8602-4
Bottles of 60 NDC 68788-8602-6
Bottles of 900 NDC 68788-8602-9
Bottles of 100 NDC 68788-8602-1
Bottles of 120 NDC 68788-8602-8
Store at 15°C to 30°C (59°F to 86°F) in well-closed containers; dispense in light-resistant containers.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Naproxen has been studied in patients with rheumatoid arthritis, osteoarthritis, polyarticular juvenile idiopathic arthritis, ankylosing spondylitis, tendonitis and bursitis, and acute gout. Improvement in patients treated for rheumatoid arthritis was demonstrated by a reduction in joint swelling, a reduction in duration of morning stiffness, a reduction in disease activity as assessed by both the investigator and patient, and by increased mobility as demonstrated by a reduction in walking time. Generally, response to naproxen has not been found to be dependent on age, sex, severity or duration of rheumatoid arthritis.
In patients with osteoarthritis, the therapeutic action of naproxen has been shown by a reduction in joint pain or tenderness, an increase in range of motion in knee joints, increased mobility as demonstrated by a reduction in walking time, and improvement in capacity to perform activities of daily living impaired by the disease.
In a clinical trial comparing standard formulations of naproxen 375 mg twice a day (750 mg a day) vs 750 mg twice a day (1,500 mg/day), 9 patients in the 750 mg group terminated prematurely because of adverse events. Nineteen patients in the 1,500 mg group terminated prematurely because of adverse events. Most of these adverse events were gastrointestinal events.
In clinical studies in patients with rheumatoid arthritis, osteoarthritis, and polyarticular juvenile idiopathic arthritis, naproxen has been shown to be comparable to aspirin and indomethacin in controlling the aforementioned measures of disease activity, but the frequency and severity of the milder gastrointestinal adverse effects (nausea, dyspepsia, heartburn) and nervous system adverse effects (tinnitus, dizziness, lightheadedness) were less in naproxen-treated patients than in those treated with aspirin or indomethacin.
In patients with ankylosing spondylitis, naproxen has been shown to decrease night pain, morning stiffness and pain at rest. In double-blind studies the drug was shown to be as effective as aspirin, but with fewer side effects.
In patients with acute gout, a favorable response to naproxen was shown by significant clearing of inflammatory changes (e.g., decrease in swelling, heat) within 24 to 48 hours, as well as by relief of pain and tenderness.
Naproxen has been studied in patients with mild to moderate pain secondary to postoperative, orthopedic, postpartum episiotomy and uterine contraction pain and dysmenorrhea. Onset of pain relief can begin within 1 hour in patients taking naproxen. Analgesic effect was shown by such measures as reduction of pain intensity scores, increase in pain relief scores, decrease in numbers of patients requiring additional analgesic medication, and delay in time to remedication. The analgesic effect has been found to last for up to 12 hours.
Naproxen may be used safely in combination with gold salts and/or corticosteroids; however, in controlled clinical trials, when added to the regimen of patients receiving corticosteroids, it did not appear to cause greater improvement over that seen with corticosteroids alone. Whether naproxen has a “steroid-sparing” effect has not been adequately studied. When added to the regimen of patients receiving gold salts, naproxen did result in greater improvement. Its use in combination with salicylates is not recommended because there is evidence that aspirin increases the rate of excretion of naproxen and data are inadequate to demonstrate that naproxen and aspirin produce greater improvement over that achieved with aspirin alone. In addition, as with other NSAIDs, the combination may result in higher frequency of adverse events than demonstrated for either product alone.
In 51Cr blood loss and gastroscopy studies with normal volunteers, daily administration of 1,000 mg of naproxen has been demonstrated to cause statistically significantly less gastric bleeding and erosion than 3,250 mg of aspirin.
SPL MEDGUIDE SECTION
|
Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | ||
|---|---|---|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. | ||
|
What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)? NSAIDs can cause serious side effects, including: | ||
|
• o o | ||
|
Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG)." Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack. | ||
|
• o o o | ||
|
The risk of getting an ulcer or bleeding increases with: o o | ||
|
o o o o |
o o o o | |
|
NSAIDs should only be used: o o o | ||
|
What are NSAIDs? NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain. | ||
|
Who should not take NSAIDs? Do not take NSAIDs: • • | ||
|
Before taking NSAIDS, tell your healthcare provider about all of your medical conditions, including if you: • • • • • **Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements.**NSAIDs and some other medicines can interact with each other and cause serious side effects.Do not start taking any new medicine without talking to your healthcare provider first. | ||
|
What are the possible side effects of NSAIDs? ** NSAIDs can cause serious side effects, including:** ** See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?”** • • • • • • • | ||
|
• Get emergency help right away if you get any of the following symptoms: | ||
|
• • • |
• • | |
|
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms: | ||
|
• • • • • • • |
• • • • • | |
|
If you take too much of your NSAID, call your healthcare provider or get medical help right away. Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
|
Other information about NSAIDs • • | ||
|
General information about the safe and effective use of NSAIDs Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals. | ||
|
**Manufactured by:**ScieGen Pharmaceuticals Inc, Hauppauge, NY 11788, USA For more information, call 1-855-724-3436 |
Revised: 4/2023
Repackaged By: Preferred Pharmaceuticals Inc.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of NSAIDs, including naproxen tablets can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of naproxen tablets use between about 20 and 30 weeks of gestation, and avoid naproxen tablets use at about 30 weeks of gestation and later in pregnancy (see Clinical Considerations, Data).
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including naproxen tablets at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In animal reproduction studies in rats, rabbits, and mice no evidence of teratogenicity or fetal harm when naproxen was administered during the period of organogenesis at doses 0.13, 0.26, and 0.6 times the maximum recommended human daily dose of 1,500 mg/day, respectively [ see Data]. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as naproxen, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including naproxen can cause premature closure of the fetal ductus arteriosus (see Data).
Oligohydramnios/Neonatal Renal Impairment:
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If naproxen tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue naproxen tablets, and follow up according to clinical practice (see Data).
Labor or Delivery
There are no studies on the effects of naproxen tablets during labor or delivery. In animal studies, NSAIDS, including naproxen, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth.
Data
Human Data
There is some evidence to suggest that when inhibitors of prostaglandin synthesis are used to delay preterm labor, there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus, and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction, and abnormal prostaglandin E levels in preterm infants. Because of the known effects of nonsteroidal anti- inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly starting at 30-weeks of gestation, or third trimester) should be avoided.
Premature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Animal data
Reproduction studies have been performed in rats at 20 mg/kg/day (0.13 times the maximum recommended human daily dose of 1,500 mg/day based on body surface area comparison), rabbits at 20 mg/kg/day (0.26 times the maximum recommended human daily dose, based on body surface area comparison), and mice at 170 mg/kg/day (0.6 times the maximum recommended human daily dose based on body surface area comparison) with no evidence of impaired fertility or harm to the fetus due to the drug.
8.2 Lactation
Risk Summary
The naproxen anion has been found in the milk of lactating women at a
concentration equivalent to approximately 1% of maximum naproxen concentration
in plasma.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for naproxen tablets and any potential adverse effects on the breastfed infant from the naproxen or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including naproxen tablets, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAIDs, including naproxen tablets, in women who have difficulties conceiving or who are undergoing investigation of infertility.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established. Pediatric dosing recommendations for polyarticular juvenile idiopathic arthritis are based on well-controlled studies [ see Dosage and Administration (2)]. There are no adequate effectiveness or dose- response data for other pediatric conditions, but the experience in polyarticular juvenile idiopathic arthritis and other use experience have established that single doses of 2.5 mg/kg to 5 mg/kg as naproxen suspension, with total daily dose not exceeding 15 mg/kg/day, are well tolerated in pediatric patients over 2 years of age.
8.5 Geriatric Use
The hepatic and renal tolerability of long-term naproxen administration was studied in two double-blind clinical trials involving 586 patients. Of the patients studied, 98 patients were age 65 and older and 10 of the 98 patients were age 75 and older. NAPROXEN was administered at doses of 375 mg twice daily or 750 mg twice daily for up to 6 months. Transient abnormalities of laboratory tests assessing hepatic and renal function were noted in some patients, although there were no differences noted in the occurrence of abnormal values among different age groups.
Elderly patients, compared to younger patients, are at greater risk for NSAID- associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [ see Warnings and Precautions (5.1, 5.2, 5.3, 5.6, 5.14)].
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. The clinical significance of this finding is unclear, although it is possible that the increase in free naproxen concentration could be associated with an increase in the rate of adverse events per a given dosage in some elderly patients. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
Experience indicates that geriatric patients may be particularly sensitive to certain adverse effects of nonsteroidal anti-inflammatory drugs. Elderly or debilitated patients seem to tolerate peptic ulceration or bleeding less well when these events do occur. Most spontaneous reports of fatal GI events are in the geriatric population [ see Warnings and Precautions (5.2)].
Naproxen is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [ see Clinical Pharmacology (12.3)]. Geriatric patients may be at a greater risk for the development of a form of renal toxicity precipitated by reduced prostaglandin formation during administration of nonsteroidal anti-inflammatory drugs [ see Warnings and Precautions (5.6)].
8.6 Hepatic Impairment
Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose [ see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance <30 mL/min) [ see Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of naproxen Tablets in women who have difficulties conceiving. (8.3)
Renal Impairment:Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance <30 mL/min). (8.7)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A 2-year study was performed in rats to evaluate the carcinogenic potential of naproxen at rat doses of 8, 16, and 24 mg/kg/day (0.05, 0.1, and 0.16 times the maximum recommended human daily dose [MRHD] of 1,500 mg/day based on a body surface area comparison). No evidence of tumorigenicity was found.
Mutagenesis
Naproxen tested positive in the in vivo sister chromatid exchange assay for but was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test).
Impairment of Fertility
Male rats were treated with 2, 5, 10, and 20 mg/kg naproxen by oral gavage for 60 days prior to mating and female rats were treated with the same doses for 14 days prior to mating and for the first 7 days of pregnancy. There were no adverse effects on fertility noted (up to 0.13 times the MRDH based on body surface area).
