Gemfibrozil
Gemfibrozil Tablets, USP 600 mg
5501438d-5247-4f2a-8e15-085276d01725
HUMAN PRESCRIPTION DRUG LABEL
Sep 16, 2025
PD-Rx Pharmaceuticals, Inc.
DUNS: 156893695
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Gemfibrozil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx Only
GEMFIBROZIL
Tablets, USP
600 mg

DESCRIPTION SECTION
DESCRIPTION
Gemfibrozil, USP is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. Each tablet also contains the following inactive ingredients: colloidal silicon dioxide, NF; croscarmellose sodium, NF; calcium stearate, NF; microcrystalline cellulose, NF; methylcellulose, USP and opadry white. The chemical name is 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid, with the following structural formula:
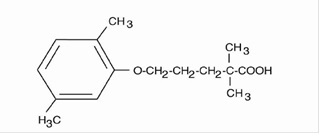
The empirical formula is C 15H 22O 3 and the molecular weight is 250.35; the solubility in water and acid is 0.0019% and in dilute base it is greater than 1%. The melting point is 58° to 61°C. Gemfibrozil is a white solid which is stable under ordinary conditions.
