Registrants (1)
402460324
Manufacturing Establishments (1)
The J. Molner Company LLC
The J. Molner Company OU
005320536
Products (1)
Hydrocortisone Butyrate
83148-012
ANDA209556
ANDA (C73584)
TOPICAL
November 30, 2023
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Hydrocortisone Butyrate Lotion, 0.1% contains hydrocortisone butyrate, a non- fluorinated hydrocortisone ester, for topical use.
Hydrocortisone butyrate is a corticosteroid.
The chemical name of hydrocortisone butyrate is Pregn-4-ene-3,20-dione, 11,21-dihydroxy-17-[(1-oxobutyl)oxy(11β)-]. It has the following structural formula:
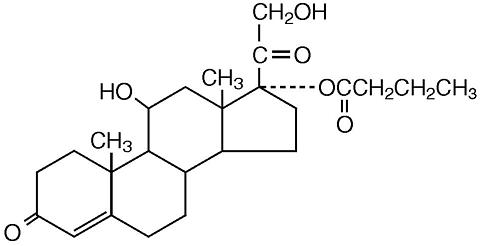
Hydrocortisone butyrate is a white to off-white powder with a molecular weight of 432.56, and a molecular formula of C 25H 36O 6. It is practically insoluble in water, slightly soluble in ether, soluble in methanol, alcohol, and acetone, and freely soluble in chloroform.
Each gram of Hydrocortisone Butyrate Lotion contains 1 mg of hydrocortisone butyrate in a white to off-white lotion base consisting of anhydrous citric acid, ceteth-20, cetostearyl alcohol, butylated hydroxytoluene (BHT), butylparaben, light mineral oil, propylparaben, purified water, sodium citrate, and white petrolatum.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in atopic dermatitis is unknown.
12.2 Pharmacodynamics
Pharmacodynamics of Hydrocortisone Butyrate Lotion is unknown.
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
Eighty-four (84) pediatric subjects (3 months to less than 18 years of age) with moderate to severe atopic dermatitis affecting at least 25% of body surface area (BSA) treated with Hydrocortisone Butyrate Lotion three times daily for up to 4 weeks were assessed for HPA axis suppression. The disease severity (moderate to severe atopic dermatitis) and the dosing regimen (three times daily) in this HPA axis trial were different from the subject population (mild to moderate atopic dermatitis) and the dosing regimen (twice daily) for which Hydrocortisone Butyrate Lotion is indicated. Seven of the 82 evaluable subjects (8.5%) demonstrated laboratory evidence of HPA axis suppression, where the criterion for defining HPA axis suppression was a serum cortisol level of less than or equal to 18 mcg/dL after cosyntropin stimulation. Subjects with HPA axis suppression ranged from 1 to 12 years of age and, at the time of enrollment, had 35% to 90% BSA involvement. These subjects did not develop any other signs or symptoms of HPA axis suppression. At the first follow-up visit, approximately 1 month after the conclusion of treatment, cosyntropin stimulation results of all subjects had returned to normal, with the exception of one subject. This last subject recovered adrenal function by the second post-treatment visit, 55 days post-treatment [seeWarnings and Precautions (5.1), Use in Specific Populations (8.4)].
12.3 Pharmacokinetics
No studies were conducted to determine the pharmacokinetics of Hydrocortisone Butyrate Lotion.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed through normal intact skin. Inflammation and/or other disease processes in the skin, occlusive dressings, or widespread application may increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids.
WARNINGS AND PRECAUTIONS SECTION
Highlight: • Endocrine System Adverse Reactions: (5)
o Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur, with the potential for glucocorticosteroid insufficiency. Consider periodic evaluations for HPA axis suppression if Hydrocortisone Butyrate Lotion is applied to large surface areas or used under occlusion. If HPA axis suppression is noted, reduce the application frequency, discontinue use, or switch to a lower potency corticosteroid. (5.1, 8.4) (5)
o Systemic effects of topical corticosteroids may also include manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria. (5.1, 8.4) (5)
o Pediatric patients may be more susceptible to systemic toxicity due to their larger skin-surface-to-body-mass ratios. (5.1, 8.4) (5)
• Ophthalmic Adverse Reactions: Topical corticosteroids, including Hydrocortisone Butyrate Lotion, may increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.2) (5)
(5)
• Skin Infections: Initiate appropriate therapy if concomitant skin infections develop. (5.3) (5)
5 WARNINGS AND PRECAUTIONS
5.1 Endocrine System Adverse Reactions
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
Use of topical corticosteroids, including Hydrocortisone Butyrate Lotion, can cause systemic adverse reactions including reversible hypothalamic-pituitary- adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. Factors that predispose a patient to HPA axis suppression include the use of high-potency steroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age. Such patients should be considered for periodic evaluation of the HPA axis. This may be done by using cosyntropin (ACTH 1-24) stimulation testing (CST). If HPA axis suppression is noted, reduce the frequency of application or discontinue Hydrocortisone Butyrate Lotion, or substitute with a less potent corticosteroid. Signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids [see Adverse Reactions (6)].
Studies conducted in pediatric subjects demonstrated reversible HPA axis suppression after use of Hydrocortisone Butyrate Lotion. Pediatric patients may be more susceptible than adults to systemic toxicity from equivalent doses of Hydrocortisone Butyrate Lotion due to their larger skin-surface-to-body- mass ratios [see Use in Specific Populations (8.4)].
Cushing's Syndrome, Hyperglycemia, and Glucosuria
Systemic adverse reactions of topical corticosteroids, including Hydrocortisone Butyrate Lotion, may also include manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria.
Additional Considerations for Endocrine Adverse Reactions
Use of more than one corticosteroid-containing product at the same time may increase total systemic corticosteroid exposure.
Minimize systemic corticosteroid adverse reactions by mitigating the risk factors for increased systemic absorption and using Hydrocortisone Butyrate Lotion as recommended [see Dosage and Administration (2)].
5.2 Ophthalmic Adverse Reactions
Use of topical corticosteroids, including Hydrocortisone Butyrate Lotion, may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported in post-marketing experience with the use of topical corticosteroid products [see Adverse Reactions (6.2)].
Avoid contact of Hydrocortisone Butyrate Lotion with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
5.3 Skin Infections
Use of topical corticosteroids, including Hydrocortisone Butyrate Lotion, may delay healing or worsen concomitant skin infections. If skin infections are present or develop, an appropriate antimicrobial agent should be used. If a favorable response does not occur promptly, use of Hydrocortisone Butyrate Lotion should be discontinued until the infection has been adequately controlled [seeAdverse Reactions (6)].
5.4 Allergic Contact Dermatitis
Use of topical corticosteroids, including Hydrocortisone Butyrate Lotion, can cause allergic contact dermatitis [see Adverse Reactions (6)]. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noticing a clinical exacerbation. Such an observation should be corroborated with appropriate patch testing. Discontinue Hydrocortisone Butyrate Lotion if the diagnosis is established .
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Patients using Hydrocortisone Butyrate Lotion should receive the following information and instructions:
Administration Instructions
Instruct patients to apply a thin layer to the affected skin two times daily and rub in gently [ see Dosage and Administration (2)].
Advise patients to discontinue Hydrocortisone Butyrate Lotion when control is achieved [ see Dosage and Administration (2)].
Advise patients to avoid use for longer than 2 weeks. Instruct patients to contact their healthcare provider if no improvement is seen within 2 weeks [ see Dosage and Administration (2)].
Advise patients toNOT[see Dosage and Administration (2) and Warnings and Precautions (5.1)]:
- Bandage, otherwise cover, or wrap the affected skin area unless directed by their healthcare provider.
- Use Hydrocortisone Butyrate Lotion in the diaper area, as diapers or plastic pants may constitute occlusive dressings.
- Use Hydrocortisone Butyrate Lotion on the face, underarms, or groin areas unless directed by their healthcare provider.
Endocrine System Adverse Reactions
Instruct patients not to use other corticosteroid-containing products while using Hydrocortisone Butyrate Lotion without first consulting their healthcare provider [see Warnings and Precautions (5.1)].
Ophthalmic Adverse Reactions
Advise patients to avoid contact with the eyes. Instruct patients to report any visual symptoms to their healthcare providers [see Warnings and Precautions (5.4)].
Lactation
Advise breastfeeding women to use Hydrocortisone Butyrate Lotion on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise to wash off prior to breastfeeding any Hydrocortisone Butyrate Lotion that has been applied to the areas at risk for direct infant contact. [see Use in Specific Populations (8.2)].
Distributed by:
The J. Molner Company LLC,
Jersey City, NJ 07302
Revised: 11/2023
PI012
