Emtricitabine and Tenofovir Disoproxil Fumarate
These highlights do not include all the information needed to use EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE TABLETS safely and effectively. See full prescribing information for EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE TABLETS. EMTRICITABINE and TENOFOVIR Disoproxil Fumarate Tablets, for oral use Initial U.S. Approval: 2004
d3b849a7-219d-4acc-9e29-8f246cb4a7a9
HUMAN PRESCRIPTION DRUG LABEL
Mar 1, 2023
Proficient Rx LP
DUNS: 079196022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Emtricitabine and Tenofovir Disoproxil Fumarate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Treatment of HIV-1 Infection
Emtricitabine and tenofovir disoproxil fumarate tablets are indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 17 kg [see Clinical Studies (14)].
1.2 HIV-1 Pre-Exposure Prophylaxis (PrEP)
Emtricitabine and tenofovir disoproxil fumarate tablets are indicated in at- risk adults and adolescents weighing at least 35 kg for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 infection. Individuals must have a negative HIV-1 test immediately prior to initiating emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
HIV-1 Treatment (1.1)
Emtricitabine and tenofovir disoproxil fumarate tablets are a two-drug combination of emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF), both HIV-1 nucleoside analog reverse transcriptase inhibitors, and is indicated:
•
in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 17 kg.
HIV-1 PrEP (1.2):
•
Emtricitabine and tenofovir disoproxil fumarate tablets is indicated in at-risk adults and adolescents weighing at least 35 kg for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 infection. Individuals must have a negative HIV-1 test immediately prior to initiating emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP is contraindicated in individuals with unknown or positive HIV-1 status [see Warnings and Precautions (5.2)].
Emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP is contraindicated in individuals with unknown or positive HIV-1 status. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Individuals with HBV
Infection
All individuals should be tested for the presence of chronic hepatitis B virus (HBV) before or when initiating emtricitabine and tenofovir disoproxil fumarate [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in HBV-infected individuals who have discontinued emtricitabine and tenofovir disoproxil fumarate. Individuals infected with HBV who discontinue emtricitabine and tenofovir disoproxil fumarate should be closely monitored with both clinical and laboratory follow- up for at least several months after stopping treatment. If appropriate, anti- hepatitis B therapy may be warranted, especially in individuals with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure. HBV-uninfected individuals should be offered vaccination.
5.2 Comprehensive Management to Reduce the Risk of Sexually Transmitted
Infections, Including HIV-1, and Development of HIV-1 Resistance When Emtricitabine and Tenofovir Disoproxil Fumarate Is Used for HIV-1 PrEP
Use emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP to reduce the risk of HIV-1 infection as part of a comprehensive prevention strategy that includes other prevention measures, including adherence to daily administration and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs). The time from initiation of emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP to maximal protection against HIV-1 infection is unknown.
Risk for HIV-1 acquisition includes behavioral, biological, or epidemiologic factors including but not limited to condomless sex, past or current STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high prevalence area or network.
Counsel individuals on the use of other prevention measures (e.g., consistent and correct condom use, knowledge of partner(s)’ HIV-1 status, including viral suppression status, regular testing for STIs that can facilitate HIV-1 transmission). Inform uninfected individuals about and support their efforts in reducing sexual risk behavior.
Use emtricitabine and tenofovir disoproxil fumarate to reduce the risk of acquiring HIV-1 only in individuals confirmed to be HIV-negative**.**HIV-1 resistance substitutions may emerge in individuals with undetected HIV-1 infection who are taking only emtricitabine and tenofovir disoproxil fumarate, because emtricitabine and tenofovir disoproxil fumarate alone does not constitute a complete regimen for HIV-1 treatment [see Microbiology (12.4)]; therefore, care should be taken to minimize the risk of initiating or continuing emtricitabine and tenofovir disoproxil fumarate before confirming the individual is HIV-1 negative.
•
Some HIV-1 tests only detect anti-HIV antibodies and may not identify HIV-1 during the acute stage of infection. Prior to initiating emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP, ask seronegative individuals about recent (in past month) potential exposure events (e.g., condomless sex or condom breaking during sex with a partner of unknown HIV-1 status or unknown viremic status, or a recent STI), and evaluate for current or recent signs or symptoms consistent with acute HIV-1 infection (e.g., fever, fatigue, myalgia, skin rash).
•
If recent (<1 month) exposures to HIV-1 are suspected or clinical symptoms consistent with acute HIV-1 infection are present, use a test approved or cleared by the FDA as an aid in the diagnosis of acute or primary HIV-1 infection.
While using emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP, HIV-1 testing should be repeated at least every 3 months, and upon diagnosis of any other STIs.
•
If an HIV-1 test indicates possible HIV-1 infection, or if symptoms consistent with acute HIV-1 infection develop following a potential exposure event, convert the HIV-1 PrEP regimen to an HIV treatment regimen until negative infection status is confirmed using a test approved or cleared by the FDA as an aid in the diagnosis of acute or primary HIV-1 infection.
Counsel HIV-1 uninfected individuals to strictly adhere to the once daily emtricitabine and tenofovir disoproxil fumarate dosing schedule. The effectiveness of emtricitabine and tenofovir disoproxil fumarate in reducing the risk of acquiring HIV-1 is strongly correlated with adherence, as demonstrated by measurable drug levels in clinical trials of emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP. Some individuals, such as adolescents, may benefit from more frequent visits and counseling to support adherence [see Use in Specific Populations (8.4), Microbiology (12.4), and Clinical Studies (14.3 and 14.4)].
5.3 New Onset or Worsening Renal Impairment
Emtricitabine and tenofovir are principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of TDF, a component of emtricitabine and tenofovir disoproxil fumarate [see Adverse Reactions (6.2)].
Prior to initiation and during use of emtricitabine and tenofovir disoproxil fumarate, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all individuals. In individuals with chronic kidney disease, also assess serum phosphorus.
Emtricitabine and tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs [NSAIDs]) [see Drug Interactions (7.1)]. Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on TDF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures, and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in individuals at risk of renal dysfunction.
Treatment of HIV-1 Infection
Dosing interval adjustment of emtricitabine and tenofovir disoproxil fumarate and close monitoring of renal function are recommended in all patients with estimated creatinine clearance 30 to 49 mL/min [see Dosage and Administration (2.6)]. No safety or efficacy data are available in patients with renal impairment who received emtricitabine and tenofovir disoproxil fumarate using these dosing guidelines, so the potential benefit of emtricitabine and tenofovir disoproxil fumarate therapy should be assessed against the potential risk of renal toxicity. Emtricitabine and tenofovir disoproxil fumarate is not recommended in patients with estimated creatinine clearance below 30 mL/min or patients requiring hemodialysis.
HIV-1 PrEP
Emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP is not recommended in uninfected individuals with estimated creatinine clearance less than 60 mL/min. If a decrease in estimated creatinine clearance is observed while using emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use [see Dosage and Administration (2.6)].
5.4 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in HIV-1 infected patients treated with combination antiretroviral therapy, including emtricitabine and tenofovir disoproxil fumarate. During the initial phase of combination antiretroviral treatment, HIV-1 infected patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
5.5 Bone Loss and Mineralization Defects
Bone Mineral Density
In clinical trials in HIV-1 infected adults and in a clinical trial of HIV-1 uninfected individuals, TDF (a component of emtricitabine and tenofovir disoproxil fumarate) was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators [see Adverse Reactions (6.1)]. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving TDF.
Clinical trials evaluating TDF in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the TDF-treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in adolescent subjects aged 12 years to less than 18 years treated for chronic hepatitis B. In all pediatric trials, skeletal growth (height) appeared to be unaffected.
The effects of TDF-associated changes in BMD and biochemical markers on long- term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adult and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial. If bone abnormalities are suspected, appropriate consultation should be obtained.
Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with TDF use [see Adverse Reactions (6.1)]. Arthralgia and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving TDF-containing products [see Warnings and Precautions (5.3)].
5.6 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including FTC and TDF, components of emtricitabine and tenofovir disoproxil fumarate, alone or in combination with other antiretrovirals. Treatment with emtricitabine and tenofovir disoproxil fumarate should be suspended in any individual who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.7 Risk of Adverse Reactions Due to Drug Interactions
The concomitant use of emtricitabine and tenofovir disoproxil fumarate and other drugs may result in known or potentially significant drug interactions, some of which may lead to possible clinically significant adverse reactions from greater exposures of concomitant drugs [see Drug Interactions (7.2)].
See Table 7 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during therapy with emtricitabine and tenofovir disoproxil fumarate; review concomitant medications during therapy with emtricitabine and tenofovir disoproxil fumarate; and monitor for adverse reactions associated with the concomitant drugs.
•
Comprehensive management to reduce the risk of acquiring HIV-1
when emtricitabine and tenofovir disoproxil fumarate is used for HIV-1 PrEP: Use as part of a comprehensive prevention strategy including other prevention measures; strictly adhere to dosing schedule. (5.2)
•
Management to reduce the risk of acquiring HIV-1 drug resistance
when emtricitabine and tenofovir disoproxil fumarate is used for HIV-1 PrEP: refer to full prescribing information for additional detail. (5.2)
•
New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Avoid administering emtricitabine and tenofovir disoproxil fumarate with concurrent or recent use of nephrotoxic drugs. (5.3)
•
Immune reconstitution syndrome during treatment of HIV-1 infection: May necessitate further evaluation and treatment. (5.4)
•
Decreases in bone mineral density (BMD): Consider assessment of BMD in individuals with a history of pathologic fracture or other risk factors for osteoporosis or bone loss. (5.5)
•
Lactic acidosis/severe hepatomegaly with steatosis: Discontinue emtricitabine and tenofovir disoproxil fumarate in individuals who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.6)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Important Information for Uninfected Individuals Taking Emtricitabine and Tenofovir Disoproxil Fumarate Tablets for HIV-1 PrEP
Advise HIV-uninfected individuals about the following [see Warnings and Precautions (5.2)]:
•
The need to confirm that they are HIV-negative before starting to take emtricitabine and tenofovir disoproxil fumarate tablets to reduce the risk of acquiring HIV-1.
•
That HIV-1 resistance substitutions may emerge in individuals with undetected HIV-1 infection who are taking emtricitabine and tenofovir disoproxil fumarate tablets, because emtricitabine and tenofovir disoproxil fumarate tablets alone does not constitute a complete regimen for HIV-1 treatment.
•
The importance of taking emtricitabine and tenofovir disoproxil fumarate tablets on a regular dosing schedule and strict adherence to the recommended dosing schedule to reduce the risk of acquiring HIV-1. Uninfected individuals who miss doses are at greater risk of acquiring HIV-1 than those who do not miss doses.
•
That emtricitabine and tenofovir disoproxil fumarate tablets does not prevent other sexually acquired infections and should only be used as part of a complete prevention strategy including other prevention measures.
•
To use condoms consistently and correctly to lower the chances of sexual contact with any body fluids such as semen, vaginal secretions, or blood.
•
The importance of knowing their HIV-1 status and the HIV-1 status of their partner(s).
•
The importance of virologic suppression in their partner(s) with HIV-1.
•
The need to get tested regularly for HIV-1 (at least every 3 months, or more frequently for some individuals such as adolescents) and to ask their partner(s) to get tested as well.
•
To report any symptoms of acute HIV-1 infection (flu-like symptoms) to their healthcare provider immediately.
•
That the signs and symptoms of acute infection include fever, headache, fatigue, arthralgia, vomiting, myalgia, diarrhea, pharyngitis, rash, night sweats, and adenopathy (cervical and inguinal).
•
To get tested for other sexually transmitted infections, such as syphilis, chlamydia, and gonorrhea, that may facilitate HIV-1 transmission.
•
To assess their sexual risk behavior and get support to help reduce sexual risk behavior.
Severe Acute Exacerbation of Hepatitis B in Patients Infected with HBV
Inform individuals that severe acute exacerbations of hepatitis B have been reported in patients who are infected with HBV and have discontinued emtricitabine and tenofovir disoproxil fumarate [see Warnings and Precautions (5.1)]. Advise HBV-infected individuals to not discontinue emtricitabine and tenofovir disoproxil fumarate tablets without first informing their healthcare provider.
New Onset or Worsening Renal Impairment
Inform HIV-1 infected patients and uninfected individuals that renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported in association with the use of TDF, a component of emtricitabine and tenofovir disoproxil fumarate tablets. Advise patients to avoid emtricitabine and tenofovir disoproxil fumarate tablets with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple NSAIDs) [see Warnings and Precautions (5.3)]. The dosing interval of emtricitabine and tenofovir disoproxil fumarate tablets may need adjustment in HIV-1 infected patients with renal impairment. Emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP should not be used in HIV-1 uninfected individuals if estimated creatinine clearance is less than 60 mL/min. If a decrease in estimated creatinine clearance is observed in uninfected individuals while using emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use [see Dosage and Administration (2.6)].
Immune Reconstitution Syndrome
Inform HIV-1 infected patients that in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started. It is believed that these symptoms are due to an improvement in the body’s immune response, enabling the body to fight infections that may have been present with no obvious symptoms. Advise patients to inform their healthcare provider immediately of any symptoms of infection [see Warnings and Precautions (5.4)].
Bone Loss and Mineralization Defects
Inform patients that decreases in bone mineral density have been observed with the use of TDF or emtricitabine and tenofovir disoproxil fumarate tablets. Consider bone monitoring in patients and uninfected individuals who have a history of pathologic bone fracture or at risk for osteopenia [see Warnings and Precautions (5.5)].
Lactic Acidosis and Severe Hepatomegaly
Inform HIV-1 infected patients and uninfected individuals that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Treatment with emtricitabine and tenofovir disoproxil fumarate tablets should be suspended in any person who develops clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.6)].
Drug Interactions
Advise individuals that emtricitabine and tenofovir disoproxil fumarate tablets may interact with many drugs; therefore, advise individuals to report to their healthcare provider the use of any other medication, including other HIV drugs and drugs for treatment of hepatitis C virus [see Warnings and Precautions (5.7) and Drug Interactions (7)].
Dosage Recommendations for Treatment of HIV-1 Infection
Inform HIV-1 infected patients that it is important to take emtricitabine and tenofovir disoproxil fumarate tablets with other antiretroviral drugs for the treatment of HIV-1 on a regular dosing schedule with or without food and to avoid missing doses as it can result in development of resistance.
Pregnancy Registry
Inform individuals using emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 treatment or HIV-1 PrEP that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to emtricitabine and tenofovir disoproxil fumarate tablets [see Use in Specific Populations (8.1)].
Lactation
Instruct mothers not to breastfeed if they are taking emtricitabine and tenofovir disoproxil fumarate tablets for the treatment of HIV-1 infection or if acute HIV-1 infection is suspected in a mother taking emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP because of the risk of passing the HIV-1 virus to the baby. In HIV-uninfected women, the benefits and risks of emtricitabine and tenofovir disoproxil fumarate tablets while breastfeeding should be evaluated, including the risk of HIV-1 acquisition due to medication nonadherence and subsequent mother to child transmission [see Use in Specific Populations (8.2)].
All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
**Dispense with Medication Guide available at: **www.aurobindousa.com/medication-guides
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Repackaged by:
Proficient Rx LP
Thousand Oaks, CA 91320
Revised: 11/2022
DESCRIPTION SECTION
11 DESCRIPTION
Emtricitabine and tenofovir disoproxil fumarate tablets are fixed-dose combination tablets containing emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF). FTC is a synthetic nucleoside analog of cytidine. TDF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. Both FTC and tenofovir exhibit inhibitory activity against HIV-1 reverse transcriptase.
Emtricitabine: The chemical name of FTC is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. FTC is the (-) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
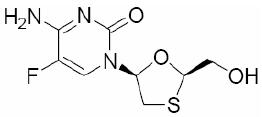
FTC is a white to off-white powder with a solubility of approximately 112 mg/mL in water at 25°C. The partition coefficient (log p) for emtricitabine is -0.43 and the pKa is 2.65.
Tenofovir Disoproxil Fumarate: TDF is a fumaric acid salt of the bis- isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir DF is 9-[(R)-2 [[bis[[(isopropoxycarbonyl)oxy]-methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
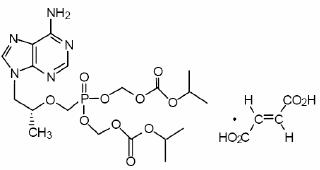
Tenofovir disoproxil fumarate is a white to off-white, crystalline powder with a solubility of 13.4 mg/mL in water at 25°C. The partition coefficient (log p) for tenofovir disoproxil is 1.25 and the pKa is 3.75. All dosages are expressed in terms of TDF except where otherwise noted.
Emtricitabine and tenofovir disoproxil fumarate tablets are for oral administration. Each film-coated tablet contains 200 mg of FTC and 300 mg of TDF (which is equivalent to 245 mg of tenofovir disoproxil), as active ingredients. The tablets also include the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, lactose monohydrate, pregelatinized starch (gluten free), and magnesium stearate. The tablets are coated with Opadry II White 32K18425, which contains lactose monohydrate, hypromellose 15cP, titanium dioxide, and triacetin.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Emtricitabine and tenofovir disoproxil fumarate is a fixed-dose combination of antiviral drugs FTC and TDF [see Microbiology (12.4)].
12.3 Pharmacokinetics
Emtricitabine and tenofovir disoproxil fumarate: One emtricitabine and tenofovir disoproxil fumarate tablet was comparable to one FTC capsule (200 mg) plus one TDF tablet (300 mg) following single-dose administration to fasting healthy subjects (N=39).
Emtricitabine: The pharmacokinetic properties of FTC are summarized in Table 8. Following oral administration of FTC, FTC is rapidly absorbed with peak plasma concentrations occurring at 1 to 2 hours postdose. Less than 4% of FTC binds to human plasma proteins in vitro, and the binding is independent of concentration over the range of 0.02 to 200 mcg/mL. Following administration of radiolabelled FTC, approximately 86% is recovered in the urine and 13% is recovered as metabolites. The metabolites of FTC include 3′-sulfoxide diastereomers and their glucuronic acid conjugate. Emtricitabine is eliminated by a combination of glomerular filtration and active tubular secretion. Following a single oral dose of FTC, the plasma FTC half-life is approximately 10 hours.
Tenofovir Disoproxil Fumarate: The pharmacokinetic properties of TDF are summarized in Table 8. Following oral administration of TDF, maximum tenofovir serum concentrations are achieved in 1.0 + 0.4 hour. Less than 0.7% of tenofovir binds to human plasma proteins in vitro, and the binding is independent of concentration over the range of 0.01 to 25 mcg/mL. Approximately 70 to 80% of the intravenous dose of tenofovir is recovered as unchanged drug in the urine. Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion. Following a single oral dose of TDF, the terminal elimination half-life of tenofovir is approximately 17 hours.
Table 4 Single Dose Pharmacokinetic Parameters for FTC and Tenofovir in Adultsa|
FTC |
Tenofovir | |
|---|---|---|
|
Fasted Oral Bioavailabilityb (%) |
92 (83.1 to 106.4) |
25 (NC to 45.0) |
|
Plasma Terminal Elimination Half-Lifeb (hr) |
10 (7.4 to 18.0) |
17 (12.0 to 25.7) |
|
Cmaxc (mcg/mL) |
1.8 ± 0.72d |
0.30 ± 0.09 |
|
AUCc (mcg•hr/mL) |
10.0 ± 3.12d |
2.29 ± 0.69 |
|
CL/Fc (mL/min) |
302 ± 94 |
1043 ± 115 |
|
CLrenalc (mL/min) |
213 ± 89 |
243 ± 33 |
|
a. NC=Not calculated |
Effects of Food on Oral Absorption
Emtricitabine and tenofovir disoproxil fumarate may be administered with or without food. Administration of emtricitabine and tenofovir disoproxil fumarate following a high fat meal (784 kcal; 49 grams of fat) or a light meal (373 kcal; 8 grams of fat) delayed the time of tenofovir Cmax by approximately 0.75 hour. The mean increases in tenofovir AUC and Cmax were approximately 35% and 15%, respectively, when administered with a high fat or light meal, compared to administration in the fasted state. In previous safety and efficacy trials, TDF (tenofovir) was taken under fed conditions. FTC systemic exposures (AUC and Cmax) were unaffected when emtricitabine and tenofovir disoproxil fumarate was administered with either a high fat or a light meal.
Specific Populations
Race
Emtricitabine: No pharmacokinetic differences due to race have been identified following the administration of FTC.
Tenofovir Disoproxil Fumarate: There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations following the administration of TDF.
Gender
Emtricitabine and Tenofovir Disoproxil Fumarate: FTC and tenofovir pharmacokinetics are similar in male and female subjects.
Pediatric Patients
Treatment of HIV-1 Infection: The pharmacokinetic data for tenofovir and FTC following administration of emtricitabine and tenofovir disoproxil fumarate in pediatric subjects weighing 17 kg and above are not available. The dosage recommendations of emtricitabine and tenofovir disoproxil fumarate in this population are based on the dosage recommendations of FTC and TDF in this population. Refer to the EMTRIVA and VIREAD prescribing information for pharmacokinetic information on the individual products in pediatric patients.
HIV-1 PrEP: The pharmacokinetic data for tenofovir and FTC following administration of emtricitabine and tenofovir disoproxil fumarate in HIV-1 uninfected adolescents weighing 35 kg and above are not available. The dosage recommendations of emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP in this population are based on safety and adherence data from the ATN113 trial [see Use in Specific Populations (8.4)] and known pharmacokinetic information in HIV-infected adolescents taking TDF and FTC for treatment.
Geriatric Patients
Pharmacokinetics of FTC and tenofovir have not been fully evaluated in the elderly (65 years of age and older).
Patients with Renal Impairment
The pharmacokinetics of FTC and tenofovir are altered in subjects with renal impairment [see Warnings and Precautions (5.3)]. In adult subjects with creatinine clearance below 50 mL/min, Cmax and AUC0-∞ of FTC and tenofovir were increased. No data are available to make dosage recommendations in pediatric patients with renal impairment.
Patients with Hepatic Impairment
The pharmacokinetics of tenofovir following a 300 mg dose of TDF have been studied in non-HIV infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects. The pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate or FTC have not been studied in subjects with hepatic impairment; however, FTC is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Assessment of Drug Interactions
The steady state pharmacokinetics of FTC and tenofovir were unaffected when FTC and TDF were administered together versus each agent dosed alone.
In vitro studies and clinical pharmacokinetic drug-drug interaction trials have shown that the potential for CYP mediated interactions involving FTC and tenofovir with other medicinal products is low.
TDF is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters. When TDF is coadministered with an inhibitor of these transporters, an increase in absorption may be observed.
No clinically significant drug interactions have been observed between FTC and famciclovir, indinavir, stavudine, TDF, and zidovudine (Tables 9 and 10). Similarly, no clinically significant drug interactions have been observed between TDF and efavirenz, methadone, nelfinavir, oral contraceptives, ribavirin, or sofosbuvir in trials conducted in healthy volunteers (Tables 11 and 12).
Table 5 Drug Interactions: Changes in Pharmacokinetic Parameters for FTC in the Presence of the Coadministered Drug a|
a. All interaction trials conducted in healthy volunteers | ||||||
|
Coadministered Drug |
Dose of Coadministered Drug (mg) |
FTC |
N |
% Change of FTC Pharmacokinetic Parametersb (90% CI) | ||
|
C****max |
AUC |
C****min | ||||
|
TDF |
300 once daily |
200 once daily |
17 |
⇔ |
⇔ |
↑ 20 |
|
Zidovudine |
300 twice daily |
200 once daily |
27 |
⇔ |
⇔ |
⇔ |
|
Indinavir |
800 x 1 |
200 x 1 |
12 |
⇔ |
⇔ |
NA |
|
Famciclovir |
500 x 1 |
200 x 1 |
12 |
⇔ |
⇔ |
NA |
|
Stavudine |
40 x 1 |
200 x 1 |
6 |
⇔ |
⇔ |
NA |
|
a. All interaction trials conducted in healthy volunteers | ||||||
|
Coadministered Drug |
Dose of Coadministered Drug (mg) |
FTC |
N |
% Change of Coadministered Drug Pharmacokinetic Parameters****b | ||
|
C****max |
AUC |
C****min | ||||
|
TDF |
300 once daily |
200 once daily |
17 |
⇔ |
⇔ |
⇔ |
|
Zidovudine |
300 twice daily |
200 once daily |
27 |
↑ 17 |
↑ 13 |
⇔ |
|
Indinavir |
800 x 1 |
200 x 1 |
12 |
⇔ |
⇔ |
NA |
|
Famciclovir |
500 x 1 |
200 x 1 |
12 |
⇔ |
⇔ |
NA |
|
Stavudine |
40 x 1 |
200 x 1 |
6 |
⇔ |
⇔ |
NA |
|
a. Subjects received VIREAD 300 mg once daily. | |||||
|
Coadministered Drug |
Dose of Coadministered Drug (mg) |
N |
% Change of Tenofovir Pharmacokinetic Parameters****b | ||
|
C****max |
AUC |
C****min | |||
|
Atazanavirc |
400 once daily |
33 |
↑ 14 |
↑ 24 |
↑ 22 |
|
Atazanavir/ Ritonavirc |
300/100 once daily |
12 |
↑ 34 |
↑ 37 |
↑ 29 |
|
Darunavir/ Ritonavird |
300/100 twice daily |
12 |
↑ 24 |
↑ 22 |
↑ 37 |
|
Indinavir |
800 three times daily |
13 |
↑ 14 |
⇔ |
⇔ |
|
Ledipasvir/ Sofosbuvire,f |
90/400 once daily |
24 |
↑ 47 |
↑ 35 |
↑ 47 |
|
Ledipasvir/ Sofosbuvire,g |
23 |
↑ 64 |
↑ 50 |
↑ 59 | |
|
Ledipasvir/ Sofosbuvirh |
90/400 once daily |
15 |
↑ 79 |
↑ 98 |
↑ 163 |
|
Ledipasvir/ Sofosbuviri |
90/400 once daily |
14 |
↑ 32 |
↑ 40 |
↑ 91 |
|
Ledipasvir/ Sofosbuvirj |
90/400 once daily |
29 |
↑ 61 |
↑ 65 |
↑ 115 |
|
Lopinavir/ |
400/100 twice daily |
24 |
⇔ |
↑ 32 |
↑ 51 |
|
Saquinavir/ |
1000/100 twice daily |
35 |
⇔ |
⇔ |
↑ 23 |
|
Sofosbuvirk |
400 single dose |
16 |
↑ 25 |
⇔ |
⇔ |
|
Sofosbuvir/ |
400/100 once daily |
24 |
↑ 44 |
↑ 40 |
↑ 84 |
|
Sofosbuvir/ |
400/100 once daily |
30 |
↑ 46 |
↑ 40 |
↑ 70 |
|
Sofosbuvir/ |
400/100/100+Voxilapreviro 100 once daily |
29 |
↑ 48 |
↑ 39 |
↑ 47 |
|
Tacrolimus |
0.05 mg/kg twice daily |
21 |
↑ 13 |
⇔ |
⇔ |
|
Tipranavir/ |
500/100 twice daily |
22 |
↓ 23 |
↓ 2 |
↑ 7 |
|
750/200 twice daily (23 doses) |
20 |
↓ 38 |
↑ 2 |
↑ 14 |
No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with emtricitabine and tenofovir disoproxil fumarate: abacavir, didanosine (buffered tablets), FTC, entecavir, and lamivudine.
Table 8 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drug in the Presence of Tenofovir|
Coadministered Drug |
Dose of Coadministered Drug (mg) |
N |
% Change of Coadministered Drug Pharmacokinetic Parameters****a | ||
|
C****max |
AUC |
C****min | |||
|
Abacavir |
300 once |
8 |
↑ 12 |
⇔ |
NA |
|
Atazanavirb |
400 once daily |
34 |
↓ 21 |
↓ 25 |
↓ 40 |
|
Atazanavirb |
Atazanavir/Ritonavir 300/100 once daily |
10 |
↓ 28 |
↓ 25c |
↓ 23c |
|
Darunavird |
Darunavir/Ritonavir 300/100 once daily |
12 |
↑ 16 |
↑ 21 |
↑ 24 |
|
Didanosinee |
250 once, simultaneously with TDF and a light mealf |
33 |
↓ 20g |
⇔g |
NA |
|
Emtricitabine |
200 once daily |
17 |
⇔ |
⇔ |
↑ 20 |
|
Indinavir |
800 three times daily x 7 days |
12 |
↓ 11 |
⇔ |
⇔ |
|
Entecavir |
1 once daily |
28 |
⇔ |
↑ 13 |
⇔ |
|
Lamivudine |
150 twice daily |
15 |
↓ 24 |
⇔ |
⇔ |
|
Lopinavir |
Lopinavir/Ritonavir 400/100 twice daily |
24 |
⇔ |
⇔ |
⇔ |
|
Saquinavir |
Saquinavir/Ritonavir |
32 |
↑ 22 |
↑ 29h |
↑ 47h |
|
Ritonavir |
⇔ |
⇔ |
↑ 23 | ||
|
Tacrolimus |
0.05 mg/kg twice daily x 7 days |
21 |
⇔ |
⇔ |
⇔ |
|
Tipranaviri |
Tipranavir/Ritonavir 500/100 twice daily |
22 |
↓ 17 |
↓ 18 |
↓ 21 |
|
Tipranavir/Ritonavir 750/200 twice daily |
20 |
↓ 11 |
↓ 9 |
↓ 12 | |
|
a. Increase = ↑; Decrease = ↓; No Effect = ⇔; NA = Not Applicable |
12.4 Microbiology
Mechanism of Action
Emtricitabine: FTC, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate (FTC- TP), which inhibits the activity of the HIV-1 reverse transcriptase (RT) by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. FTC-TP is a weak inhibitor of mammalian DNA polymerases α, β, ε and mitochondrial DNA polymerase γ.
Tenofovir Disoproxil Fumarate: TDF is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. TDF requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate (TFV-DP), which inhibits the activity of HIV-1 RT by competing with the natural substrate deoxyadenosine 5'-triphosphate and, after incorporation into DNA, by DNA chain termination. TFV-DP is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Antiviral Activity
Emtricitabine and Tenofovir Disoproxil Fumarate: No antagonism was observed in combination studies evaluating the cell culture antiviral activity of FTC and tenofovir together.
Emtricitabine: The antiviral activity of FTC against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, the MAGI-CCR5 cell line, and peripheral blood mononuclear cells. The 50% effective concentration (EC50) values for FTC were in the range of 0.0013 to 0.64 μM (0.0003 to 0.158 mcg/mL). In drug combination studies of FTC with nucleoside RT inhibitors (abacavir, lamivudine, stavudine, zidovudine), non-nucleoside RT inhibitors (delavirdine, efavirenz, nevirapine), and protease inhibitors (amprenavir, nelfinavir, ritonavir, saquinavir), no antagonism was observed. Emtricitabine displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 0.007 to 0.075 μM) and showed strain-specific activity against HIV-2 (EC50 values ranged from 0.007 to 1.5 μM).
Tenofovir Disoproxil Fumarate: The antiviral activity of tenofovir against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, primary monocyte/macrophage cells, and peripheral blood lymphocytes. The EC50 values for tenofovir were in the range of 0.04 to 8.5 μM. In drug combination studies of tenofovir with nucleoside RT inhibitors (abacavir, didanosine, lamivudine, stavudine, zidovudine), non-nucleoside RT inhibitors (delavirdine, efavirenz, nevirapine), and protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir), no antagonism was observed. Tenofovir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.5 to 2.2 μM) and showed strain-specific activity against HIV-2 (EC50 values ranged from 1.6 μM to 5.5 μM).
Prophylactic Activity in a Nonhuman Primate Model of HIV-1 Transmission
Emtricitabine and Tenofovir Disoproxil Fumarate: The prophylactic activity of the combination of daily oral FTC and TDF was evaluated in a controlled study of macaques inoculated once weekly for 14 weeks with SIV/HIV-1 chimeric virus (SHIV) applied to the rectal surface. Of the 18 control animals, 17 became infected after a median of 2 weeks. In contrast, 4 of the 6 animals treated daily with oral FTC and TDF remained uninfected and the two infections that did occur were significantly delayed until 9 and 12 weeks and exhibited reduced viremia. An M184I-expressing FTC-resistant variant emerged in 1 of the 2 macaques after 3 weeks of continued drug exposure.
Resistance
Emtricitabine and Tenofovir Disoproxil Fumarate: HIV-1 isolates with reduced susceptibility to the combination of FTC and tenofovir have been selected in cell culture. Genotypic analysis of these isolates identified the M184V/I and/or K65R amino acid substitutions in the viral RT. In addition, a K70E substitution in the HIV-1 RT has been selected by tenofovir and results in reduced susceptibility to tenofovir.
In Study 934, a clinical trial of treatment-naïve subjects [see Clinical Studies (14.2)], resistance analysis was performed on HIV-1 isolates from all confirmed virologic failure subjects with greater than 400 copies/mL of HIV-1 RNA at Week 144 or early discontinuation. Development of efavirenz resistance- associated substitutions occurred most frequently and was similar between the treatment arms. The M184V amino acid substitution, associated with resistance to FTC and lamivudine, was observed in 2/19 analyzed subject isolates in the FTC+TDF group and in 10/29 analyzed subject isolates in the zidovudine/lamivudine group. Through 144 weeks of Study 934, no subjects have developed a detectable K65R or K70E substitution in their HIV-1 as analyzed through standard genotypic analysis.
Emtricitabine: FTC-resistant isolates of HIV-1 have been selected in cell culture and in vivo. Genotypic analysis of these isolates showed that the reduced susceptibility to FTC was associated with a substitution in the HIV-1 RT gene at codon 184 which resulted in an amino acid substitution of methionine by valine or isoleucine (M184V/I).
Tenofovir Disoproxil Fumarate: HIV-1 isolates with reduced susceptibility to tenofovir have been selected in cell culture. These viruses expressed a K65R substitution in RT and showed a 2-to 4-fold reduction in susceptibility to tenofovir.
In treatment-naïve subjects, isolates from 8/47 (17%) analyzed subjects developed the K65R substitution in the TDF arm through 144 weeks; 7 occurred in the first 48 weeks of treatment and 1 at Week 96. In treatment-experienced subjects, 14/304 (5%) isolates from subjects failing TDF through Week 96 showed greater than 1.4-fold (median 2.7) reduced susceptibility to tenofovir. Genotypic analysis of the resistant isolates showed a K65R amino acid substitution in the HIV-1 RT.
iPrEx Trial: In the iPrEx trial, a clinical trial of HIV-1 seronegative adult subjects [see Clinical Studies (14.3)], no amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion among 48 subjects in the emtricitabine and tenofovir disoproxil fumarate group and 83 subjects in the placebo group who became infected with HIV-1 during the trial. Ten subjects were observed to be HIV-1 infected at time of enrollment. The M184V/I substitutions associated with resistance to FTC were observed in 3 of the 10 subjects (2 of 2 in the emtricitabine and tenofovir disoproxil fumarate group and 1 of 8 in the placebo group). One of the two subjects in the emtricitabine and tenofovir disoproxil fumarate group harbored wild type virus at enrollment and developed the M184V substitution 4 weeks after enrollment. The other subject had indeterminate resistance at enrollment but was found to have the M184I substitution 4 weeks after enrollment.
Partners PrEP Trial: In the Partners PrEP trial, a clinical trial of HIV-1 seronegative adult subjects [see Clinical Studies (14.4)], no variants expressing amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion among 12 subjects in the emtricitabine and tenofovir disoproxil fumarate group, 15 subjects in the TDF group, and 51 subjects in the placebo group. Fourteen subjects were observed to be HIV-1 infected at the time of enrollment (3 in the emtricitabine and tenofovir disoproxil fumarate group, 5 in the TDF group, and 6 in the placebo group). One of the three subjects in the emtricitabine and tenofovir disoproxil fumarate group who was infected with wild type virus at enrollment selected an M184V expressing virus by Week 12. Two of the five subjects in the TDF group had tenofovir-resistant viruses at the time of seroconversion; one subject infected with wild type virus at enrollment developed a K65R substitution by Week 16, while the second subject had virus expressing the combination of D67N and K70R substitutions upon seroconversion at Week 60, although baseline virus was not genotyped and it is unclear if the resistance emerged or was transmitted. Following enrollment, 4 subjects (2 in the TDF group, 1 in the emtricitabine and tenofovir disoproxil fumarate group, and 1 in the placebo group) had virus expressing K103N or V106A substitutions, which confer high-level resistance to NNRTIs but have not been associated with FTC or TDF and may have been present in the infecting virus.
ATN113 Trial: In ATN113, a clinical trial of HIV-1 seronegative adolescent subjects [see Use in Specific Populations (8.4)], no amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion from any of the 3 subjects who became infected with HIV-1 during the trial. All 3 subjects who seroconverted were nonadherent to the recommended emtricitabine and tenofovir disoproxil fumarate dosage.
Cross Resistance
Emtricitabine and Tenofovir Disoproxil Fumarate: Cross-resistance among certain NRTIs has been recognized. The M184V/I and/or K65R substitutions selected in cell culture by the combination of FTC and tenofovir are also observed in some HIV-1 isolates from subjects failing treatment with tenofovir in combination with either FTC or lamivudine, and either abacavir or didanosine. Therefore, cross-resistance among these drugs may occur in patients whose virus harbors either or both of these amino acid substitutions.
Emtricitabine: FTC-resistant isolates (M184V/I) were cross-resistant to lamivudine but retained susceptibility in cell culture to the NRTIs didanosine, stavudine, tenofovir, and zidovudine, and to NNRTIs (delavirdine, efavirenz, and nevirapine). HIV-1 isolates containing the K65R substitution, selected in vivo by abacavir, didanosine, and tenofovir, demonstrated reduced susceptibility to inhibition by FTC. Viruses harboring substitutions conferring reduced susceptibility to stavudine and zidovudine (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E), or didanosine (L74V) remained sensitive to FTC. HIV-1 containing the K103N substitution associated with resistance to NNRTIs was susceptible to FTC.
Tenofovir Disoproxil Fumarate: The K65R and K70E substitutions selected by tenofovir are also selected in some HIV-1 infected patients treated with abacavir or didanosine. HIV-1 isolates with the K65R and K70E substitutions also showed reduced susceptibility to FTC and lamivudine. Therefore, cross- resistance among these NRTIs may occur in patients whose virus harbors the K65R or K70E substitutions. HIV-1 isolates from subjects (N=20) whose HIV-1 expressed a mean of 3 zidovudine-associated RT amino acid substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) showed a 3.1-fold decrease in the susceptibility to tenofovir. Subjects whose virus expressed an L74V substitution without zidovudine resistance-associated substitutions (N=8) had reduced response to TDF. Limited data are available for patients whose virus expressed a Y115F substitution (N=3), Q151M substitution (N=2), or T69 insertion (N=4), all of whom had a reduced response.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Emtricitabine: In long-term oral carcinogenicity studies of FTC, no drug- related increases in tumor incidence were found in mice at doses up to 750 mg/kg/day (26 times the human systemic exposure at the therapeutic dose of 200 mg/day) or in rats at doses up to 600 mg/kg/day (31 times the human systemic exposure at the therapeutic dose).
FTC was not genotoxic in the reverse mutation bacterial test (Ames test), or the mouse lymphoma or mouse micronucleus assays.
FTC did not affect fertility in male rats at approximately 140-fold or in male and female mice at approximately 60-fold higher exposures (AUC) than in humans given the recommended 200 mg daily dose. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60-fold higher than human exposures at the recommended 200 mg daily dose.
Tenofovir Disoproxil Fumarate: Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the high dose in female mice, liver adenomas were increased at exposures 16 times that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 5 times that observed in humans at the therapeutic dose.
TDF was mutagenic in the in vitro mouse lymphoma assay and negative in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, TDF was negative when administered to male mice.
There were no effects on fertility, mating performance, or early embryonic development when TDF was administered to male rats at a dose equivalent to 10 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 15 days prior to mating through day 7 of gestation. There was, however, an alteration of the estrous cycle in female rats.
13.2 Animal Toxicology and/or Pharmacology
Tenofovir and TDF administered in toxicology studies to rats, dogs, and monkeys at exposures (based on AUCs) greater than or equal to 6-fold those observed in humans caused bone toxicity. In monkeys the bone toxicity was diagnosed as osteomalacia. Osteomalacia observed in monkeys appeared to be reversible upon dose reduction or discontinuation of tenofovir. In rats and dogs, the bone toxicity manifested as reduced bone mineral density. The mechanism(s) underlying bone toxicity is unknown.
Evidence of renal toxicity was noted in four animal species. Increases in serum creatinine, BUN, glycosuria, proteinuria, phosphaturia, and/or calciuria and decreases in serum phosphate were observed to varying degrees in these animals. These toxicities were noted at exposures (based on AUCs) 2 to 20 times higher than those observed in humans. The relationship of the renal abnormalities, particularly the phosphaturia, to the bone toxicity is not known.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of Emtricitabine and Tenofovir Disoproxil
Fumarate Tablets for Treatment of HIV-1 Infection or for HIV-1 PrEP
Prior to or when initiating emtricitabine and tenofovir disoproxil fumarate tablets, test individuals for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation, and during use of emtricitabine and tenofovir disoproxil fumarate tablets, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all individuals. In individuals with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.3)].
2.2 HIV-1 Screening for Individuals Receiving Emtricitabine and Tenofovir
Disoproxil Fumarate Tablets for HIV-1 PrEP
Screen all individuals for HIV-1 infection immediately prior to initiating emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP and at least once every 3 months while taking emtricitabine and tenofovir disoproxil fumarate tablets, and upon diagnosis of any other sexually transmitted infections (STIs) [see Indications and Usage (1.2), Contraindications (4), and Warnings and Precautions (5.2)].
If recent (<1 month) exposures to HIV-1 are suspected or clinical symptoms consistent with acute HIV-1 infection are present, use a test approved or cleared by the FDA as an aid in the diagnosis of acute or primary HIV-1 infection [see Warnings and Precautions (5.2), Use in Specific Populations (8.4), and Clinical Studies (14.3 and 14.4)].
2.3 Recommended Dosage for Treatment of HIV-1 Infection in Adults and
Pediatric Patients Weighing at Least 35 kg
Emtricitabine and tenofovir disoproxil fumarate tablets are a two-drug fixed dose combination product containing emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF). The recommended dosage of emtricitabine and tenofovir disoproxil fumarate tablets in adults and in pediatric patients weighing at least 35 kg is one tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food [see Clinical Pharmacology (12.3)].
2.4 Recommended Dosage for Treatment of HIV-1 Infection in Pediatric
Patients Weighing at Least 17 kg and Able to Swallow a Tablet
The recommended oral dosage of emtricitabine and tenofovir disoproxil fumarate tablets for pediatric patients weighing at least 17 kg and who can swallow a tablet is presented in Table 1. Tablets should be taken once daily with or without food. Weight should be monitored periodically and the emtricitabine and tenofovir disoproxil fumarate tablets dose adjusted accordingly.
Table 1 Dosing for Treatment of HIV-1 Infection in Pediatric Patients Weighing 17 kg to less than 35 kg|
Body Weight (kg) |
Dosing of Emtricitabine and Tenofovir Disoproxil Fumarate Tablets (FTC/TDF) |
|
17 to less than 22 |
one 100 mg /150 mg tablet once daily |
|
22 to less than 28 |
one 133 mg /200 mg tablet once daily |
|
28 to less than 35 |
one 167 mg /250 mg tablet once daily |
2.5 Recommended Dosage for HIV-1 PrEP in Adults and Adolescents Weighing at
Least 35 kg
The dosage of emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP is one tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food in HIV-1 uninfected adults and adolescents weighing at least 35 kg [see Clinical Pharmacology (12.3)].
2.6 Dosage Adjustment in Individuals with Renal Impairment
Treatment of HIV-1 Infection
Table 2 provides dosage interval adjustment for patients with renal impairment. No dosage adjustment is necessary for HIV-1 infected patients with mild renal impairment (creatinine clearance 50 to 80 mL/min). The safety and effectiveness of the dosing interval adjustment recommendations in patients with moderate renal impairment (creatinine clearance 30 to 49 mL/min) have not been clinically evaluated; therefore, clinical response to treatment and renal function should be closely monitored in these patients [see Warnings and Precautions (5.3)].
No data are available to make dosage recommendations in pediatric patients with renal impairment.
Table 2 Dosage Interval Adjustment for HIV-1 Infected Adult Patients with Altered Creatinine Clearance|
a. Calculated using ideal (lean) body weight | |||
|
**Creatinine Clearance (mL/min)**a | |||
|
**>**50 |
30 to 49 |
<30 (Including Patients Requiring Hemodialysis) | |
|
Recommended Dosing Interval |
Every 24 hours |
Every 48 hours |
Emtricitabine and tenofovir disoproxil fumarate tablets are not recommended. |
HIV-1 PrEP
Emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP is not recommended in HIV-1 uninfected individuals with estimated creatinine clearance below 60 mL/min [see Warnings and Precautions (5.3)].
If a decrease in estimated creatinine clearance is observed in uninfected individuals while using emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use [see Warnings and Precautions (5.3)].
•
Testing: Prior to or when initiating emtricitabine and tenofovir disoproxil fumarate tablets test for hepatitis B virus infection. Prior to initiation and during use of emtricitabine and tenofovir disoproxil fumarate tablets, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all individuals. In individuals with chronic kidney disease, also assess serum phosphorus. (2.1)
•
HIV-1 Screening: Screen all individuals for HIV-1 infection immediately prior to initiating emtricitabine and tenofovir disoproxil fumarate tablets for HIV-1 PrEP and at least once every 3 months while taking emtricitabine and tenofovir disoproxil fumarate tablets, and upon diagnosis of any other sexually transmitted infections (STIs). (2.2)
Treatment of HIV-1 Infection
•
Recommended dosage in adults and pediatric patients weighing at least 35 kg: One emtricitabine and tenofovir disoproxil fumarate tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food. (2.3)
•
Recommended dosage in pediatric patients weighing at least 17 kg: One emtricitabine and tenofovir disoproxil fumarate low-strength tablet (100 mg/150 mg, 133 mg/200 mg, or 167 mg/250 mg based on body weight) once daily taken orally with or without food. (2.4)
•
Recommended dosage in renally impaired HIV-1 infected adult patients:
o
Creatinine clearance (CrCl) 30 to 49 mL/min: 1 tablet every 48 hours. (2.6)
o
CrCl below 30 mL/min or hemodialysis: Emtricitabine and tenofovir disoproxil fumarate tablets are not recommended. (2.6)
HIV-1 Pre-Exposure Prophylaxis (PrEP)
•
Recommended dosage in HIV-1 uninfected adults and adolescents weighing at least 35 kg: One emtricitabine and tenofovir disoproxil fumarate tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food. (2.5)
•
Recommended dosage in renally impaired HIV-uninfected individuals: Emtricitabine and tenofovir disoproxil fumarate tablets are not recommended in HIV-uninfected individuals if CrCl is below 60 mL/min. (2.6)
