Blood Liquescence
DRUG FACTS:
42f57741-8d47-4e50-a8fb-adfc73453ed2
HUMAN OTC DRUG LABEL
Sep 22, 2025
Nutritional Specialties, Inc.
DUNS: 032744609
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Allium Cepa, Hyssopus Officinalis, Avena Sativa, Paloondo, Urtica Dioica, Trifolium Pratense, Zingiber Officinale, Capsicum Annuum, Hepar (Bovine), Hypophysis Suis, Juglans Regia, Medulla Ossis Suis, Remex Crispus, Spleen (Bovine), Camphora, Magnesia Phosphorica, Ferrum Phosphoricum, Pyrogenium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (20)
Drug Labeling Information
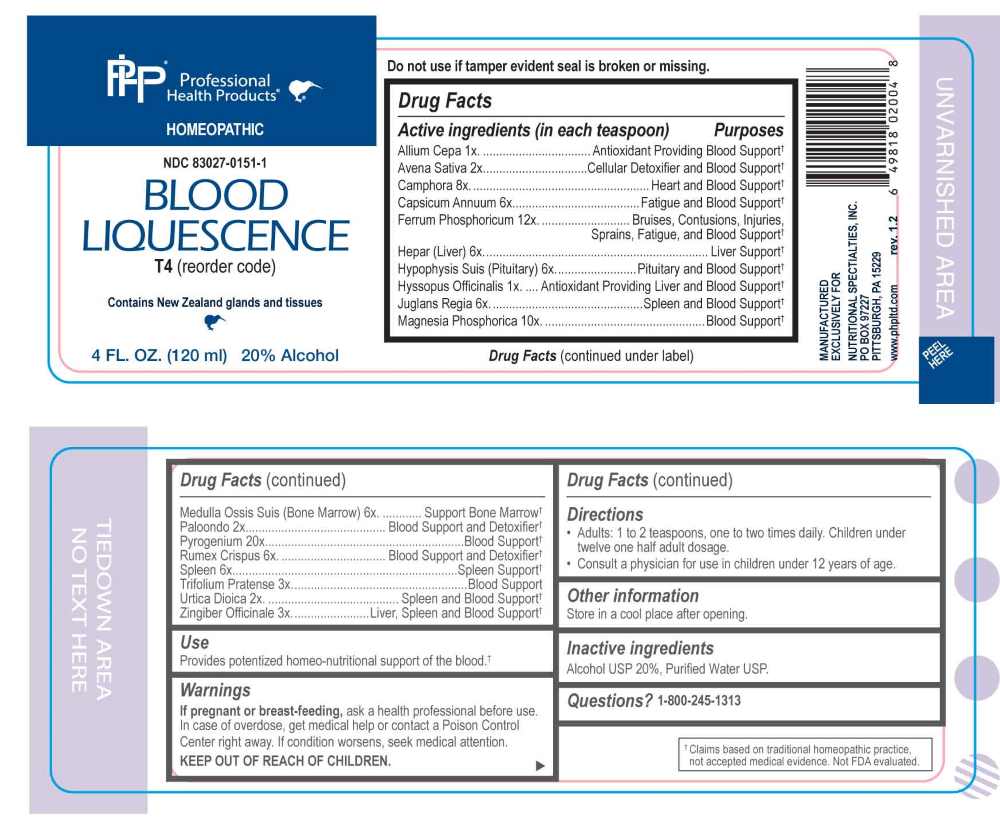
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL DISPLAY:
Professional Health Products
HOMEOPATHIC
NDC 83027-0151-1
BLOOD LIQUESCENCE
4 FL. OZ. (120 ml)
INDICATIONS & USAGE SECTION
USES:
Provides potentized homeo-nutritional support of the blood.†
†Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENTS:
Allium Cepa 1X, Avena Sativa 2X, Camphora 8X, Capsicum Annuum 6X, Ferrum Phosphoricum 12X, Hepar 6X, Hypophysis Suis 6X, Hyssopus Officinalis 1X, Juglans Regia 6X, Magnesia Phosphorica 10X, Medulla Ossis Suis 6X, Paloondo 2X, Pyrogenium 20X, Remex Crispus 6X, Spleen 6X, Trifolium Pratense 3X, Urtica Dioica 2X, Zingiber Officinale 3X.
OTC - PURPOSE SECTION
PURPOSE:
Allium Cepa – Antioxidant Providing Blood Support,† Avena Sativa – Cellular Detoxifier and Blood Support,† Camphora – Heart and Blood Support,† Capsicum Annuum – Fatigue and Blood Support,† Ferrum Phosphoricum – Bruises, Contusions, Injuries, Sprains, Fatigue, and Blood Support,† Hepar - Liver Support,† Hypophysis Suis – Pituitary and Blood Support,† Hyssopus Officinalis – Antioxidant Providing Liver and Blood Support,† Juglans Regia – Spleen and Blood Support,† Magnesia Phosphorica – Blood Support,† Medulla Ossis Suis – Support Bone Marrow,† Paloondo – Blood Support and Detoxifier, Pyrogenium – Blood Support, Remex Crispus - Blood Support and Detoxifier, Spleen – Spleen Support, Trifolium Pratense – Blood Support,† Urtica Dioica – Spleen and Blood Support,† Zingiber Officinale – Liver, Spleen and Blood Support†
†Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS SECTION
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
If condition worsens, seek medical attention.
KEEP OUT OF REACH OF CHILDREN
Do not use if tamper evident seal is broken or missing.
Store in a cool place after opening
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS:
• Adults: 1 to 2 teaspoons, one to two times daily. Children under twelve one half adult dosage.
• Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENT SECTION
INACTIVE INGREDIENTS:
Alcohol USP 20%, Purified Water USP.
OTC - QUESTIONS SECTION
QUESTIONS:
MANUFACTURED EXCLUSIVELY FOR
NUTRITIONAL SPECIALTIES, INC.
PO BOX 97227
PITTSBURGH, PA 15229
www.phpitd.com
