Docetaxel
These highlights do not include all the information needed to use DOCETAXEL INJECTION safely and effectively. See full prescribing information for DOCETAXEL INJECTION. DOCETAXEL injection, for intravenous use Initial U.S. Approval: 1996
00c61d11-d6b7-4618-b6f6-2592dbcc8af7
HUMAN PRESCRIPTION DRUG LABEL
Mar 7, 2024
Hospira, Inc.
DUNS: 141588017
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DOCETAXEL ANHYDROUS
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
DOCETAXEL ANHYDROUS
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
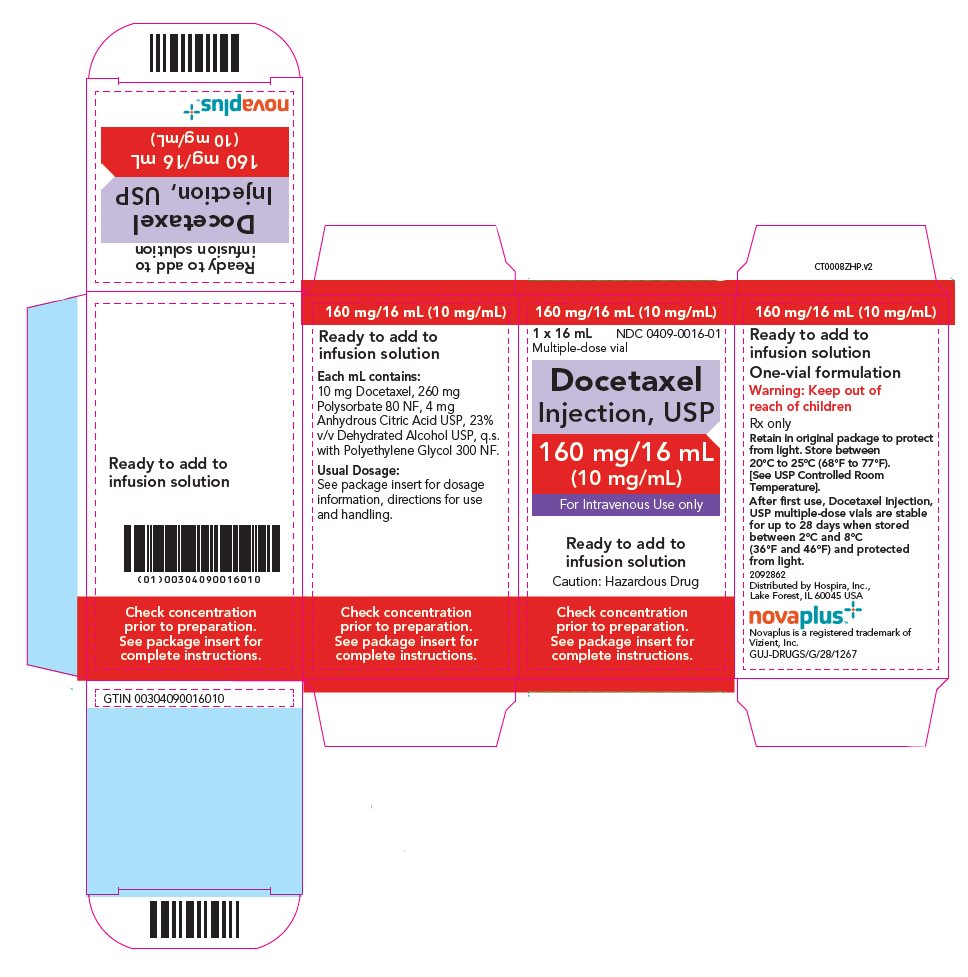
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 16 mL Vial Carton
160 mg/16 mL (10 mg/mL)
NDC 0409-0016-01
1 x 16 mL
Multiple-dose vial
Docetaxel
Injection, USP
160 mg/16 mL
(10 mg/mL)
For Intravenous Use only
Ready to add to
infusion solution
Caution: Hazardous Drug
Check concentration
prior to preparation.
See package insert for
complete instructions.
Boxed Warning section
WARNING: TOXIC DEATHS, HEPATOTOXICITY, NEUTROPENIA, HYPERSENSITIVITY
REACTIONS, and FLUID RETENTION
See full prescribing information for complete boxed warning.
•
**Treatment-related mortality increases with abnormal liver function, at higher doses, and in patients with NSCLC and prior platinum-based therapy receiving docetaxel at 100 mg/m****2**** (****5.1****)**
•
**Avoid use of Docetaxel Injection if bilirubin > ULN, or if AST and/or ALT >1.5 × ULN concomitant with alkaline phosphatase >2.5 × ULN. LFT elevations increase risk of severe or life-threatening complications. Obtain LFTs before each treatment cycle (****5.2****)**
•
**Do not administer Docetaxel Injection to patients with neutrophil counts <1500 cells/mm****3****. Obtain frequent blood counts to monitor for neutropenia (****4****,****5.3****)**
•
**Severe hypersensitivity, including fatal anaphylaxis, has been reported in patients who received dexamethasone premedication. Severe reactions require immediate discontinuation of Docetaxel Injection and administration of appropriate therapy (****5.5****)**
•
**Contraindicated if history of severe hypersensitivity reactions to docetaxel or to drugs formulated with polysorbate 80 (****4****)**
•
**Severe fluid retention may occur despite dexamethasone (****5.6****)**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Breast Cancer
Docetaxel Injection is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of prior chemotherapy.
Docetaxel Injection in combination with doxorubicin and cyclophosphamide is indicated for the adjuvant treatment of patients with operable node-positive breast cancer.
1.2 Non-small Cell Lung Cancer
Docetaxel Injection as a single agent is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of prior platinum-based chemotherapy.
Docetaxel Injection in combination with cisplatin is indicated for the treatment of patients with unresectable, locally advanced or metastatic non- small cell lung cancer who have not previously received chemotherapy for this condition.
1.3 Prostate Cancer
Docetaxel Injection in combination with prednisone is indicated for the treatment of patients with metastatic castration-resistant prostate cancer.
1.4 Gastric Adenocarcinoma
Docetaxel Injection in combination with cisplatin and fluorouracil is indicated for the treatment of patients with advanced gastric adenocarcinoma, including adenocarcinoma of the gastroesophageal junction, who have not received prior chemotherapy for advanced disease.
1.5 Head and Neck Cancer
Docetaxel Injection in combination with cisplatin and fluorouracil is indicated for the induction treatment of patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN).
Docetaxel Injection is a microtubule inhibitor indicated for:
•
**Breast Cancer (BC):** single agent for locally advanced or metastatic BC after chemotherapy failure; and with doxorubicin and cyclophosphamide as adjuvant treatment of operable node-positive BC (1.1)
•
**Non-small Cell Lung Cancer (NSCLC):** single agent for locally advanced or metastatic NSCLC after platinum therapy failure; and with cisplatin for unresectable, locally advanced or metastatic untreated NSCLC (1.2)
•
**Castration-Resistant Prostate Cancer (CRPC):** with prednisone in metastatic castration-resistant prostate cancer (1.3)
•
**Gastric Adenocarcinoma (GC):** with cisplatin and fluorouracil for untreated, advanced GC, including the gastroesophageal junction (1.4)
•
**Squamous Cell Carcinoma of the Head and Neck (SCCHN):** with cisplatin and fluorouracil for induction treatment of locally advanced SCCHN (1.5)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Docetaxel Injection is contraindicated in patients with:
•
neutrophil counts of <1500 cells/mm3 [see Warnings and Precautions (5.3)].
•
a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Severe reactions, including anaphylaxis, have occurred [see Warnings and Precautions (5.5)].
•
Hypersensitivity to docetaxel or polysorbate 80 (4)
•
Neutrophil counts of <1500 cells/mm3 (4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Docetaxel is a CYP3A4 substrate. In vitro studies have shown that the metabolism of docetaxel may be modified by the concomitant administration of compounds that induce, inhibit, or are metabolized by cytochrome P450 3A4.
In vivo studies showed that the exposure of docetaxel increased 2.2-fold when it was coadministered with ketoconazole, a potent inhibitor of CYP3A4. Protease inhibitors, particularly ritonavir, may increase the exposure of docetaxel. Concomitant use of Docetaxel Injection and drugs that inhibit CYP3A4 may increase exposure to docetaxel and should be avoided. In patients receiving treatment with Docetaxel Injection close monitoring for toxicity and a Docetaxel Injection dose reduction could be considered if systemic administration of a potent CYP3A4 inhibitor cannot be avoided [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)].
•
Cytochrome P450 3A4 inducers, inhibitors, or substrates: May alter docetaxel metabolism. (7)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Docetaxel Injection, USP is a colorless to pale yellow solution available as:
•
20 mg/2 mL (10 mg/mL) single-dose vial
•
160 mg/16 mL (10 mg/mL) multiple-dose vial
•
20 mg/2 mL (10 mg/mL) single-dose vial (3)
•
160 mg/16 mL (10 mg/mL) multiple-dose vial (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal reproduction studies and its mechanism of action, Docetaxel Injection can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Available data from case reports in the literature and pharmacovigilance with docetaxel use in pregnant women are not sufficient to inform the drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Docetaxel Injection contains alcohol which can interfere with neurobehavioral development (see Clinical Considerations). In animal reproductive studies, administration of docetaxel to pregnant rats and rabbits during the period of organogenesis caused an increased incidence of embryo-fetal toxicities, including intrauterine mortality, at doses as low as 0.02 and 0.003 times the recommended human dose based on body surface area, respectively (see Data). Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, miscarriage, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Docetaxel Injection contains alcohol [see Warnings and Precautions (5.13)]. Published studies have demonstrated that alcohol is associated with fetal harm including central nervous system abnormalities, behavioral disorders, and impaired intellectual development.
Data
Animal data
Intravenous administration of ≥0.3 and 0.03 mg/kg/day docetaxel to pregnant rats and rabbits, respectively, during the period of organogenesis caused an increased incidence of intrauterine mortality, resorptions, reduced fetal weights, and fetal ossification delays. Maternal toxicity was also observed at these doses, which were approximately 0.02 and 0.003 times the daily maximum recommended human dose based on body surface area, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of docetaxel in human milk, or on its effects on milk production or the breastfed child. No lactation studies in animals have been conducted. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Docetaxel Injection and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Based on findings in animals, Docetaxel Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating Docetaxel Injection.
Contraception
Females
Based on genetic toxicity findings, advise females of reproductive potential to use effective contraception during treatment and for 2 months after the last dose of Docetaxel Injection.
Males
Based on genetic toxicity findings, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of Docetaxel Injection.
Infertility
Based on findings in animal studies, Docetaxel Injection may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The alcohol content of Docetaxel Injection should be taken into account when given to pediatric patients [see Warnings and Precautions (5.13)].
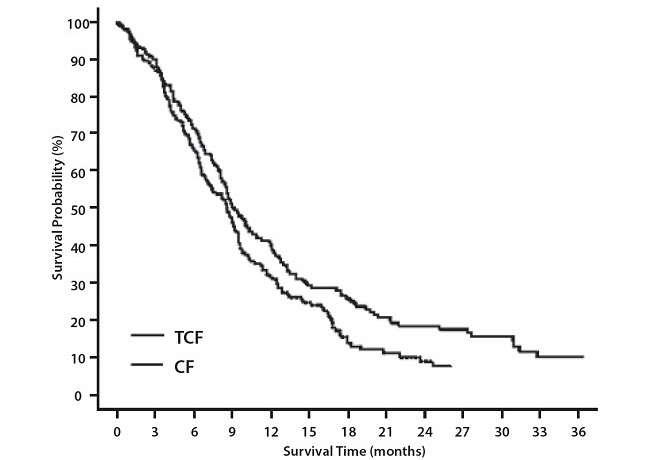
The efficacy of docetaxel in pediatric patients as monotherapy or in combination has not been established. The overall safety profile of docetaxel in pediatric patients receiving monotherapy or TCF was consistent with the known safety profile in adults.
Docetaxel has been studied in a total of 289 pediatric patients: 239 in 2 trials with monotherapy and 50 in combination treatment with cisplatin and 5-fluorouracil (TCF).
Docetaxel Monotherapy
Docetaxel monotherapy was evaluated in a dose-finding phase 1 trial in 61 pediatric patients (median age 12.5 years, range 1–22 years) with a variety of refractory solid tumors. The recommended dose was 125 mg/m2 as a 1-hour intravenous infusion every 21 days. The primary dose limiting toxicity was neutropenia.
The recommended dose for docetaxel monotherapy was evaluated in a phase 2 single-arm trial in 178 pediatric patients (median age 12 years, range 1–26 years) with a variety of recurrent/refractory solid tumors. Efficacy was not established with tumor response rates ranging from one complete response (CR) (0.6%) in a patient with undifferentiated sarcoma to four partial responses (2.2%) seen in one patient each with Ewing Sarcoma, neuroblastoma, osteosarcoma, and squamous cell carcinoma.
Docetaxel in Combination
Docetaxel was studied in combination with cisplatin and 5-fluorouracil (TCF) versus cisplatin and 5-fluorouracil (CF) for the induction treatment of nasopharyngeal carcinoma (NPC) in pediatric patients prior to chemoradiation consolidation. Seventy-five patients (median age 16 years, range 9 to 21 years) were randomized (2:1) to docetaxel (75 mg/m2) in combination with cisplatin (75 mg/m2) and 5-fluorouracil (750 mg/m2) (TCF) or to cisplatin (80 mg/m2) and 5-fluorouracil (1000 mg/m2/day) (CF). The primary endpoint was the CR rate following induction treatment of NPC. One patient out of 50 in the TCF group (2%) had a complete response while none of the 25 patients in the CF group had a complete response.
Pharmacokinetics
Pharmacokinetic parameters for docetaxel were determined in 2 pediatric solid tumor trials. Following docetaxel administration at 55 mg/m2 to 235 mg/m2 in a 1-hour intravenous infusion every 3 weeks in 25 patients aged 1 to 20 years (median 11 years), docetaxel clearance was 17.3±10.9 L/h/m2.
Docetaxel was administered in combination with cisplatin and 5-fluorouracil (TCF), at dose levels of 75 mg/m2 in a 1-hour intravenous infusion day 1 in 28 patients aged 10 to 21 years (median 16 years, 17 patients were older than 16). Docetaxel clearance was 17.9±8.75 L/h/m2, corresponding to an AUC of 4.20±2.57 μg∙h/mL.
In summary, the body surface area adjusted clearance of docetaxel monotherapy and TCF combination in children were comparable to those in adults [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in elderly patients.
Non-small Cell Lung Cancer
In a study conducted in chemotherapy-naïve patients with NSCLC (TAX326), 148 patients (36%) in the docetaxel+cisplatin group were 65 years of age or greater. There were 128 patients (32%) in the vinorelbine+cisplatin group 65 years of age or greater. In the docetaxel+cisplatin group, patients less than 65 years of age had a median survival of 10.3 months (95% CI: 9.1 months, 11.8 months) and patients 65 years or older had a median survival of 12.1 months (95% CI: 9.3 months, 14 months). In patients 65 years of age or greater treated with docetaxel+cisplatin, diarrhea (55%), peripheral edema (39%) and stomatitis (28%) were observed more frequently than in the vinorelbine+cisplatin group (diarrhea 24%, peripheral edema 20%, stomatitis 20%). Patients treated with docetaxel+cisplatin who were 65 years of age or greater were more likely to experience diarrhea (55%), infections (42%), peripheral edema (39%) and stomatitis (28%) compared to patients less than the age of 65 administered the same treatment (43%, 31%, 31% and 21%, respectively).
When docetaxel was combined with carboplatin for the treatment of chemotherapy-naïve, advanced non-small cell lung carcinoma, patients 65 years of age or greater (28%) experienced higher frequency of infection compared to similar patients treated with docetaxel+cisplatin, and a higher frequency of diarrhea, infection and peripheral edema than elderly patients treated with vinorelbine+cisplatin.
Prostate Cancer
Of the 333 patients treated with docetaxel every three weeks plus prednisone in the prostate cancer study (TAX327), 209 patients were 65 years of age or greater and 68 patients were older than 75 years. In patients treated with docetaxel every three weeks, the following treatment-emergent adverse reactions occurred at rates ≥10% higher in patients 65 years of age or greater compared to younger patients: anemia (71% vs. 59%), infection (37% vs. 24%), nail changes (34% vs. 23%), anorexia (21% vs. 10%), weight loss (15% vs. 5%), respectively.
Breast Cancer
In the adjuvant breast cancer trial (TAX316), docetaxel in combination with doxorubicin and cyclophosphamide was administered to 744 patients of whom 48 (6%) were 65 years of age or greater. The number of elderly patients who received this regimen was not sufficient to determine whether there were differences in safety and efficacy between elderly and younger patients.
Gastric Cancer
Among the 221 patients treated with Docetaxel Injection in combination with cisplatin and fluorouracil in the gastric cancer study, 54 were 65 years of age or older and 2 patients were older than 75 years. In this study, the number of patients who were 65 years of age or older was insufficient to determine whether they respond differently from younger patients. However, the incidence of serious adverse reactions was higher in the elderly patients compared to younger patients. The incidence of the following adverse reactions (all grades, regardless of relationship): lethargy, stomatitis, diarrhea, dizziness, edema, febrile neutropenia/neutropenic infection occurred at rates ≥10% higher in patients who were 65 years of age or older compared to younger patients. Elderly patients treated with TCF should be closely monitored.
Head and Neck Cancer
Among the 174 and 251 patients who received the induction treatment with Docetaxel Injection in combination with cisplatin and fluorouracil (TPF) for SCCHN in the TAX323 and TAX324 studies, 18 (10%) and 32 (13%) of the patients were 65 years of age or older, respectively.
These clinical studies of Docetaxel Injection in combination with cisplatin and fluorouracil in patients with SCCHN did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience with this treatment regimen has not identified differences in responses between elderly and younger patients.
8.6 Hepatic Impairment
Avoid Docetaxel Injection in patients with bilirubin > ULN and patients with AST and/or ALT >1.5 × ULN concomitant with alkaline phosphatase >2.5 × ULN [see Boxed Warning, Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
The alcohol content of Docetaxel Injection should be taken into account when given to patients with hepatic impairment [see Warnings and Precautions (5.13)].
•
Lactation: Advise women not to breastfeed. (8.2)
•
Females and Males of Reproductive Potential: Verify pregnancy status of females prior to initiation of Docetaxel Injection. (8.3)
OVERDOSAGE SECTION
10 OVERDOSAGE
There is no known antidote for Docetaxel Injection overdosage. In case of overdosage, the patient should be kept in a specialized unit where vital functions can be closely monitored. Anticipated complications of overdosage include: bone marrow suppression, peripheral neurotoxicity, and mucositis. Patients should receive therapeutic G-CSF as soon as possible after discovery of overdose. Other appropriate symptomatic measures should be taken, as needed.
In two reports of overdose, one patient received 150 mg/m2 and the other received 200 mg/m2 as 1-hour infusions. Both patients experienced severe neutropenia, mild asthenia, cutaneous reactions, and mild paresthesia, and recovered without incident. In mice, lethality was observed following single intravenous doses that were ≥154 mg/kg (about 4.5 times the human dose of 100 mg/m2 on a mg/m2 basis); neurotoxicity associated with paralysis, non- extension of hind limbs, and myelin degeneration was observed in mice at 48 mg/kg (about 1.5 times the human dose of 100 mg/m2 basis). In male and female rats, lethality was observed at a dose of 20 mg/kg (comparable to the human dose of 100 mg/m2 on a mg/m2 basis) and was associated with abnormal mitosis and necrosis of multiple organs.
DESCRIPTION SECTION
11 DESCRIPTION
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semisynthesis beginning with a precursor extracted from the renewable needle biomass of yew plants. The chemical name for docetaxel is (2R,3S)-N-carboxy-3-phenylisoserine,N-tert-butyl ester, 13-ester with 5β-20-epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate. Docetaxel (anhydrous) has the following structural formula:

Docetaxel is a white to almost-white powder with an empirical formula of C43H53NO14, and a molecular weight of 807.88. It is highly lipophilic and practically insoluble in water.
Docetaxel Injection, USP is a sterile, non-pyrogenic, clear, colorless to pale yellow solution at 10 mg/mL concentration.
Each mL contains 10 mg docetaxel (anhydrous), 260 mg polysorbate 80 NF, 4 mg anhydrous citric acid USP, 23% v/v dehydrated alcohol USP, and polyethylene glycol 300 NF.
Docetaxel Injection is available in single-dose vials containing 20 mg (2 mL) docetaxel (anhydrous) and multiple-dose vials containing 160 mg (16 mL) docetaxel (anhydrous).
Docetaxel Injection requires NO prior dilution with a diluent and is ready to add to the infusion solution.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Docetaxel is an antineoplastic agent that acts by disrupting the microtubular network in cells that is essential for mitotic and interphase cellular functions. Docetaxel binds to free tubulin and promotes the assembly of tubulin into stable microtubules while simultaneously inhibiting their disassembly. This leads to the production of microtubule bundles without normal function and to the stabilization of microtubules, which results in the inhibition of mitosis in cells. Docetaxel's binding to microtubules does not alter the number of protofilaments in the bound microtubules, a feature which differs from most spindle poisons currently in clinical use.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of docetaxel has been evaluated in cancer patients after administration of 20 mg/m2 to 115 mg/m2 in phase 1 studies. The area under the curve (AUC) was dose proportional following doses of 70 mg/m2 to 115 mg/m2 with infusion times of 1 to 2 hours.
Docetaxel’s pharmacokinetic profile is consistent with a three-compartment pharmacokinetic model, with initial rapid distribution phase and the late (terminal) phase.
Distribution
Mean steady state volume of distribution was 113 L. Docetaxel is approximately 94% protein bound in vitro, mainly to α1-acid glycoprotein, albumin, and lipoproteins. In three cancer patients, the in vitro binding to plasma proteins was approximately 97%. Dexamethasone does not affect the protein binding of docetaxel.
Elimination
With extended plasma sampling up to 8 to 22 days post infusion, the estimated mean total body clearance was 18 L/h/m2 (range of means: 14 to 23) and mean terminal elimination half-life was 116 hours (range of means: 92 to 135).
Metabolism
Docetaxel is metabolized by the CYP3A4 isoenzyme in vitro [see Drug Interactions (7)].
Excretion
In three cancer patients urinary and fecal excretion accounted for approximately 6% and 75% of the administered radioactivity, respectively, within 7 days. About 80% of the radioactivity recovered in feces was excreted during the first 48 hours as 1 major and 3 minor metabolites with less than 8% as unchanged drug.
Specific Populations
Effect of Age
A population pharmacokinetic analysis was carried out after docetaxel treatment of 535 patients dosed at 100 mg/m2. Pharmacokinetic parameters estimated by this analysis were very close to those estimated from phase 1 studies. The pharmacokinetics of docetaxel was not influenced by age.
Effect of Gender
The population pharmacokinetics analysis described above also indicated that gender did not influence the pharmacokinetics of docetaxel.
Hepatic Impairment
The population pharmacokinetic analysis described above indicated that in patients with clinical chemistry data suggestive of mild to moderate liver impairment (AST and/or ALT >1.5 times ULN concomitant with alkaline phosphatase >2.5 times ULN), total body clearance was lowered by an average of 27%, resulting in a 38% increase in systemic exposure (AUC). This average, however, includes a substantial range and there is, at present, no measurement that would allow recommendation for dose adjustment in such patients. Patients with combined abnormalities of transaminase and alkaline phosphatase should not be treated with Docetaxel Injection. Patients with severe hepatic impairment have not been studied [see Warnings and Precautions (5.2), Use in Specific Populations (8.6)].
Effect of Race
Mean total body clearance for Japanese patients dosed at the range of 10 mg/m2 to 90 mg/m2 was similar to that of European/American populations dosed at 100 mg/m2, suggesting no significant difference in the elimination of docetaxel in the two populations.
Drug Interaction Studies
Effect of Ketoconazole
The effect of ketoconazole (a strong CYP3A4 inhibitor) on the pharmacokinetics of docetaxel was investigated in 7 cancer patients. Patients were randomized to receive either docetaxel (100 mg/m2 intravenous) alone or docetaxel (10 mg/m2 intravenous) in combination with ketoconazole (200 mg orally once daily for 3 days) in a crossover design with a 3-week washout period. The results of this study indicated that the mean dose-normalized AUC of docetaxel was increased 2.2-fold and its clearance was reduced by 49% when docetaxel was coadministered with ketoconazole [see Dosage and Administration (2.7), Drug Interactions (7)].
Effect of Combination Therapies
•
Dexamethasone: Docetaxel total body clearance was not modified by pretreatment with dexamethasone.
•
Cisplatin: Clearance of docetaxel in combination therapy with cisplatin was similar to that previously observed following monotherapy with docetaxel. The pharmacokinetic profile of cisplatin in combination therapy with docetaxel was similar to that observed with cisplatin alone.
•
Cisplatin and Fluorouracil: The combined administration of docetaxel, cisplatin and fluorouracil in 12 patients with solid tumors had no influence on the pharmacokinetics of each individual drug.
•
Prednisone: A population pharmacokinetic analysis of plasma data from 40 patients with metastatic castration-resistant prostate cancer indicated that docetaxel systemic clearance in combination with prednisone is similar to that observed following administration of docetaxel alone.
•
Cyclophosphamide and Doxorubicin: A study was conducted in 30 patients with advanced breast cancer to determine the potential for drug-drug interactions between docetaxel (75 mg/m2), doxorubicin (50 mg/m2), and cyclophosphamide (500 mg/m2) when administered in combination. The coadministration of docetaxel had no effect on the pharmacokinetics of doxorubicin and cyclophosphamide when the three drugs were given in combination compared to coadministration of doxorubicin and cyclophosphamide only. In addition, doxorubicin and cyclophosphamide had no effect on docetaxel plasma clearance when the three drugs were given in combination compared to historical data for docetaxel monotherapy.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Locally Advanced or Metastatic Breast Cancer
The efficacy and safety of docetaxel have been evaluated in locally advanced or metastatic breast cancer after failure of previous chemotherapy (alkylating agent-containing regimens or anthracycline-containing regimens).
Randomized Trials
In one randomized trial, patients with a history of prior treatment with an anthracycline-containing regimen were assigned to treatment with docetaxel (100 mg/m2 every 3 weeks) or the combination of mitomycin (12 mg/m2 every 6 weeks) and vinblastine (6 mg/m2 every 3 weeks). Two hundred three patients were randomized to docetaxel and 189 to the comparator arm. Most patients had received prior chemotherapy for metastatic disease; only 27 patients on the docetaxel arm and 33 patients on the comparator arm entered the study following relapse after adjuvant therapy. Three-quarters of patients had measurable, visceral metastases. The primary endpoint was time to progression. The following table summarizes the study results (see Table 12).
Table 12: Efficacy of Docetaxel in the Treatment of Breast Cancer Patients Previously Treated with an Anthracycline-Containing Regimen (Intent- to-Treat Analysis)
| |||
|
Efficacy Parameter |
Docetaxel |
Mitomycin/Vinblastine |
p-value |
|
Median Survival |
11.4 months |
8.7 months |
p=0.01 |
|
Risk Ratio*, Mortality 95% CI (Risk Ratio) |
0.73 0.58–0.93 | ||
|
Median Time to Progression |
4.3 months |
2.5 months |
p=0.01 |
|
Risk Ratio*, Progression 95% CI (Risk Ratio) |
0.75 0.61–0.94 | ||
|
Overall Response Rate Complete Response Rate |
28.1% 3.4% |
9.5% 1.6% |
p<0.0001 Chi Square |
In a second randomized trial, patients previously treated with an alkylating- containing regimen were assigned to treatment with docetaxel (100 mg/m2) or doxorubicin (75 mg/m2) every 3 weeks. One hundred sixty-one patients were randomized to docetaxel and 165 patients to doxorubicin. Approximately one- half of patients had received prior chemotherapy for metastatic disease, and one-half entered the study following relapse after adjuvant therapy. Three- quarters of patients had measurable, visceral metastases. The primary endpoint was time to progression. The study results are summarized below (see Table 13).
Table 13: Efficacy of Docetaxel in the Treatment of Breast Cancer Patients Previously Treated with an Alkylating-Containing Regimen (Intent-to- Treat Analysis)
| |||
|
Efficacy Parameter |
Docetaxel |
Doxorubicin |
p-value |
|
Median Survival |
14.7 months |
14.3 months |
p=0.39 |
|
Risk Ratio*, Mortality 95% CI (Risk Ratio) |
0.89 0.68–1.16 | ||
|
Median Time to Progression |
6.5 months |
5.3 months |
p=0.45 |
|
Risk Ratio*, Progression 95% CI (Risk Ratio) |
0.93 0.71–1.16 | ||
|
Overall Response Rate Complete Response Rate |
45.3% 6.8% |
29.7% 4.2% |
p=0.004 Chi Square |
In another multicenter open-label, randomized trial (TAX313), in the treatment of patients with advanced breast cancer who progressed or relapsed after one prior chemotherapy regimen, 527 patients were randomized to receive docetaxel monotherapy 60 mg/m2 (n=151), 75 mg/m2 (n=188) or 100 mg/m2 (n=188). In this trial, 94% of patients had metastatic disease and 79% had received prior anthracycline therapy. Response rate was the primary endpoint. Response rates increased with docetaxel dose: 19.9% for the 60 mg/m2 group compared to 22.3% for the 75 mg/m2 and 29.8% for the 100 mg/m2 group; pair-wise comparison between the 60 mg/m2 and 100 mg/m2 groups was statistically significant (p=0.037).
Single Arm Studies
Docetaxel at a dose of 100 mg/m2 was studied in six single arm studies involving a total of 309 patients with metastatic breast cancer in whom previous chemotherapy had failed. Among these, 190 patients had anthracycline- resistant breast cancer, defined as progression during an anthracycline- containing chemotherapy regimen for metastatic disease, or relapse during an anthracycline-containing adjuvant regimen. In anthracycline-resistant patients, the overall response rate was 37.9% (72/190; 95% CI: 31.0–44.8) and the complete response rate was 2.1%.
Docetaxel was also studied in three single arm Japanese studies at a dose of 60 mg/m2, in 174 patients who had received prior chemotherapy for locally advanced or metastatic breast cancer. Among 26 patients whose best response to an anthracycline had been progression, the response rate was 34.6% (95% CI: 17.2–55.7), similar to the response rate in single arm studies of 100 mg/m2.
14.2 Adjuvant Treatment of Breast Cancer
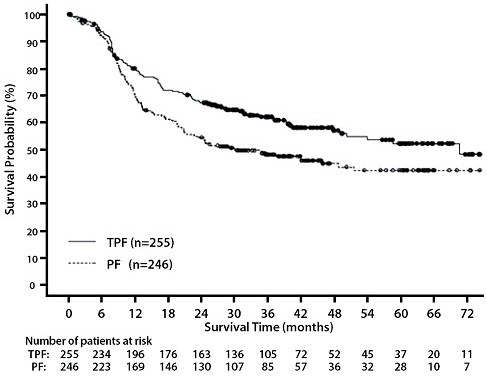
A multicenter, open-label, randomized trial (TAX316) evaluated the efficacy and safety of docetaxel for the adjuvant treatment of patients with axillary- node-positive breast cancer and no evidence of distant metastatic disease. After stratification according to the number of positive lymph nodes (1–3, 4+), 1491 patients were randomized to receive either docetaxel 75 mg/m2 administered 1-hour after doxorubicin 50 mg/m2 and cyclophosphamide 500 mg/m2 (TAC arm), or doxorubicin 50 mg/m2 followed by fluorouracil 500 mg/m2 and cyclophosphamide 500 mg/m2 (FAC arm). Both regimens were administered every 3 weeks for 6 cycles. Docetaxel was administered as a 1-hour infusion; all other drugs were given as intravenous bolus on day 1. In both arms, after the last cycle of chemotherapy, patients with positive estrogen and/or progesterone receptors received tamoxifen 20 mg daily for up to 5 years. Adjuvant radiation therapy was prescribed according to guidelines in place at participating institutions and was given to 69% of patients who received TAC and 72% of patients who received FAC.
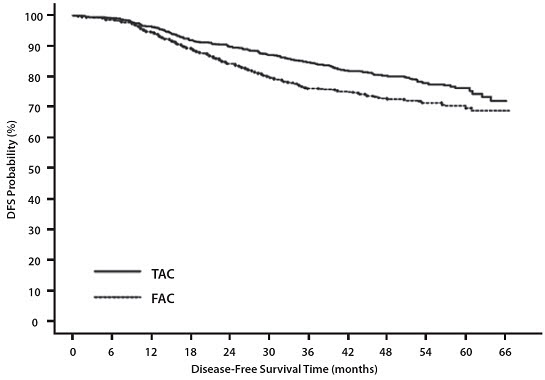
Results from a second interim analysis (median follow-up 55 months) are as follows: In study TAX316, the docetaxel-containing combination regimen TAC showed significantly longer disease-free survival (DFS) than FAC (hazard ratio=0.74; 2-sided 95% CI=0.60, 0.92, stratified log rank p=0.0047). The primary endpoint, disease-free survival, included local and distant recurrences, contralateral breast cancer and deaths from any cause. The overall reduction in risk of relapse was 25.7% for TAC-treated patients (see Figure 1).
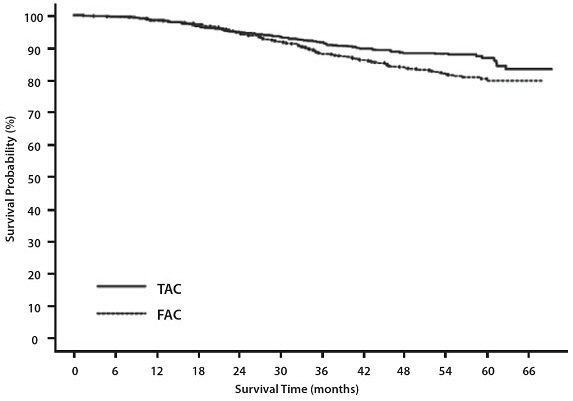
At the time of this interim analysis, based on 219 deaths, overall survival was longer for TAC than FAC (hazard ratio=0.69, 2-sided 95% CI=0.53, 0.90) (see Figure 2). There will be further analysis at the time survival data mature.
|
Figure 1 - TAX316 Disease Free Survival K-M Curve |
|---|
|
|
|
Figure 2 - TAX316 Overall Survival K-M Curve |
|---|
|
|
The following table describes the results of subgroup analyses for DFS and OS (see Table 14).
Table 14: Subset Analyses-Adjuvant Breast Cancer Study
| |||||
|
Disease Free Survival |
Overall Survival | ||||
|
Patient subset |
Number of patients |
Hazard ratio* |
95% CI |
Hazard ratio* |
95% CI |
|
No. of positive nodes | |||||
|
Overall |
744 |
0.74 |
(0.60, 0.92) |
0.69 |
(0.53, 0.90) |
|
1–3 |
467 |
0.64 |
(0.47, 0.87) |
0.45 |
(0.29, 0.70) |
|
4+ |
277 |
0.84 |
(0.63, 1.12) |
0.93 |
(0.66, 1.32) |
|
Receptor status | |||||
|
Positive |
566 |
0.76 |
(0.59, 0.98) |
0.69 |
(0.48, 0.99) |
|
Negative |
178 |
0.68 |
(0.48, 0.97) |
0.66 |
(0.44, 0.98) |
14.3 Non-small Cell Lung Cancer (NSCLC)
The efficacy and safety of docetaxel has been evaluated in patients with unresectable, locally advanced or metastatic non-small cell lung cancer whose disease has failed prior platinum-based chemotherapy or in patients who are chemotherapy-naïve.
Monotherapy with Docetaxel for NSCLC Previously Treated with Platinum-Based Chemotherapy
Two randomized, controlled trials established that a docetaxel dose of 75 mg/m2 was tolerable and yielded a favorable outcome in patients previously treated with platinum-based chemotherapy (see below). Docetaxel at a dose of 100 mg/m2, however, was associated with unacceptable hematologic toxicity, infections, and treatment-related mortality and this dose should not be used [see Boxed Warning, Dosage and Administration (2.7), Warnings and Precautions (5.3)].
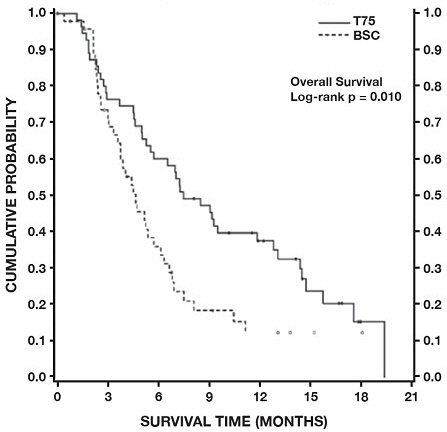
One trial (TAX317), randomized patients with locally advanced or metastatic non-small cell lung cancer, a history of prior platinum-based chemotherapy, no history of taxane exposure, and an ECOG performance status ≤2 to docetaxel or best supportive care. The primary endpoint of the study was survival. Patients were initially randomized to docetaxel 100 mg/m2 or best supportive care, but early toxic deaths at this dose led to a dose reduction to docetaxel 75 mg/m2. A total of 104 patients were randomized in this amended study to either docetaxel 75 mg/m2 or best supportive care.
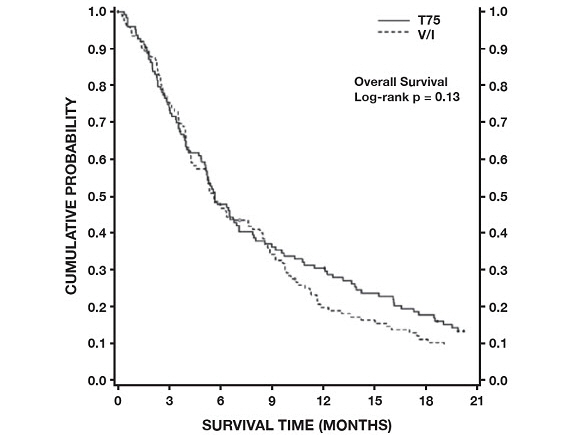
In a second randomized trial (TAX320), 373 patients with locally advanced or metastatic non-small cell lung cancer, a history of prior platinum-based chemotherapy, and an ECOG performance status ≤2 were randomized to docetaxel 75 mg/m2, docetaxel 100 mg/m2 and a treatment in which the investigator chose either vinorelbine 30 mg/m2 days 1, 8, and 15 repeated every 3 weeks or ifosfamide 2 g/m2 days 1–3 repeated every 3 weeks. Forty percent of the patients in this study had a history of prior paclitaxel exposure. The primary endpoint was survival in both trials. The efficacy data for the docetaxel 75 mg/m2 arm and the comparator arms are summarized in Table 15 and Figures 3 and 4 showing the survival curves for the two studies.
Table 15: Efficacy of Docetaxel in the Treatment of Non-small Cell Lung Cancer Patients Previously Treated with a Platinum-Based Chemotherapy Regimen (Intent-to-Treat Analysis)
| ||||
|
TAX317 |
TAX320 | |||
|
Docetaxel |
Best Supportive Care |
Docetaxel |
Control (V/I***)** | |
|
Overall Survival |
p=0.01 |
p=0.13 | ||
|
Risk Ratio†, Mortality |
0.56 |
0.82 | ||
|
Median Survival |
7.5 months‡ |
4.6 months |
5.7 months |
5.6 months |
|
% 1-year Survival |
37%ठ|
12% |
30%ठ|
20% |
|
Time to Progression |
12.3 weeks‡ |
7.0 weeks |
8.3 weeks |
7.6 weeks |
|
Response Rate |
5.5% |
Not Applicable |
5.7% |
0.8% |
Only one of the two trials (TAX317) showed a clear effect on survival, the primary endpoint; that trial also showed an increased rate of survival to one year. In the second study (TAX320) the rate of survival at one year favored docetaxel 75 mg/m2.
|
Figure 3 - TAX317 Survival K-M Curves - |
|---|
|
|
|
Figure 4 - TAX320 Survival K-M Curves - |
|---|
|
|
Patients treated with docetaxel at a dose of 75 mg/m2 experienced no deterioration in performance status and body weight relative to the comparator arms used in these trials.
Combination Therapy with Docetaxel for Chemotherapy-Naïve NSCLC
In a randomized controlled trial (TAX326), 1218 patients with unresectable stage IIIB or IV NSCLC and no prior chemotherapy were randomized to receive one of three treatments: docetaxel 75 mg/m2 as a 1 hour infusion immediately followed by cisplatin 75 mg/m2 over 30 to 60 minutes every 3 weeks; vinorelbine 25 mg/m2 administered over 6 – 10 minutes on days 1, 8, 15, 22 followed by cisplatin 100 mg/m2 administered on day 1 of cycles repeated every 4 weeks; or a combination of docetaxel and carboplatin.
The primary efficacy endpoint was overall survival. Treatment with docetaxel+cisplatin did not result in a statistically significantly superior survival compared to vinorelbine+cisplatin (see table below). The 95% confidence interval of the hazard ratio (adjusted for interim analysis and multiple comparisons) shows that the addition of docetaxel to cisplatin results in an outcome ranging from a 6% inferior to a 26% superior survival compared to the addition of vinorelbine to cisplatin. The results of a further statistical analysis showed that at least (the lower bound of the 95% confidence interval) 62% of the known survival effect of vinorelbine when added to cisplatin (about a 2-month increase in median survival; Wozniak et al. JCO, 1998) was maintained. The efficacy data for the docetaxel+cisplatin arm and the comparator arm are summarized in Table 16.
Table 16: Survival Analysis of Docetaxel in Combination Therapy for Chemotherapy-Naïve NSCLC
| ||
|
Comparison |
Docetaxel + Cisplatin n=408 |
Vinorelbine + Cisplatin n=405 |
|
Kaplan-Meier Estimate of Median Survival |
10.9 months |
10.0 months |
|
p-value* |
0.122 | |
|
Estimated Hazard Ratio† |
0.88 | |
|
Adjusted 95% CI‡ |
(0.74, 1.06) |
The second comparison in the same three-arm study, vinorelbine+cisplatin versus docetaxel+carboplatin, did not demonstrate superior survival associated with the docetaxel arm (Kaplan-Meier estimate of median survival was 9.1 months for docetaxel+carboplatin compared to 10.0 months on the vinorelbine+cisplatin arm) and the docetaxel+carboplatin arm did not demonstrate preservation of at least 50% of the survival effect of vinorelbine added to cisplatin. Secondary endpoints evaluated in the trial included objective response and time to progression. There was no statistically significant difference between docetaxel+cisplatin and vinorelbine+cisplatin with respect to objective response and time to progression (see Table 17).
Table 17: Response and TTP Analysis of Docetaxel in Combination Therapy for Chemotherapy-Naïve NSCLC
| |||
|
Endpoint |
Docetaxel + Cisplatin |
Vinorelbine + Cisplatin |
p-value |
|
Objective Response Rate |
31.6% |
24.4% |
Not Significant |
|
Median Time to Progression† |
21.4 weeks |
22.1 weeks |
Not Significant |
14.4 Castration-Resistant Prostate Cancer
The safety and efficacy of docetaxel in combination with prednisone in patients with metastatic castration-resistant prostate cancer were evaluated in a randomized multicenter active control trial. A total of 1006 patients with Karnofsky Performance Status (KPS) ≥60 were randomized to the following treatment groups:
•
Docetaxel 75 mg/m2 every 3 weeks for 10 cycles.
•
Docetaxel 30 mg/m2 administered weekly for the first 5 weeks in a 6-week cycle for 5 cycles.
•
Mitoxantrone 12 mg/m2 every 3 weeks for 10 cycles.
All 3 regimens were administered in combination with prednisone 5 mg twice daily, continuously.
In the docetaxel every three week arm, a statistically significant overall survival advantage was demonstrated compared to mitoxantrone. In the docetaxel weekly arm, no overall survival advantage was demonstrated compared to the mitoxantrone control arm. Efficacy results for the docetaxel every 3 week arm versus the control arm are summarized in Table 18 and Figure 5.
Table 18: Efficacy of Docetaxel in the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer (Intent-to-Treat Analysis)
| ||
|
Docetaxel + Prednisone |
Mitoxantrone + Prednisone | |
|
Number of patients |
335 |
337 |
|
Median survival (months) |
18.9 |
16.5 |
|
95% CI |
(17.0–21.2) |
(14.4–18.6) |
|
Hazard ratio |
0.761 |
— |
|
95% CI |
(0.619–0.936) |
— |
|
p-value* |
0.0094 |
— |
|
Figure 5 - TAX327 Survival K-M Curves |
|---|
|
|
14.5 Gastric Adenocarcinoma
A multicenter, open-label, randomized trial was conducted to evaluate the safety and efficacy of Docetaxel Injection for the treatment of patients with advanced gastric adenocarcinoma, including adenocarcinoma of the gastroesophageal junction, who had not received prior chemotherapy for advanced disease. A total of 445 patients with KPS >70 were treated with either Docetaxel Injection (T) (75 mg/m2 on day 1) in combination with cisplatin (C) (75 mg/m2 on day 1) and fluorouracil (F) (750 mg/m2 per day for 5 days) or cisplatin (100 mg/m2 on day 1) and fluorouracil (1000 mg/m2 per day for 5 days). The length of a treatment cycle was 3 weeks for the TCF arm and 4 weeks for the CF arm. The demographic characteristics were balanced between the two treatment arms. The median age was 55 years, 71% were male, 71% were Caucasian, 24% were 65 years of age or older, 19% had a prior curative surgery and 12% had palliative surgery. The median number of cycles administered per patient was 6 (with a range of 1–16) for the TCF arm compared to 4 (with a range of 1–12) for the CF arm. Time to progression (TTP) was the primary endpoint and was defined as time from randomization to disease progression or death from any cause within 12 weeks of the last evaluable tumor assessment or within 12 weeks of the first infusion of study drugs for patients with no evaluable tumor assessment after randomization. The hazard ratio (HR) for TTP was 1.47 (CF/TCF, 95% CI: 1.19–1.83) with a significantly longer TTP (p=0.0004) in the TCF arm. Approximately 75% of patients had died at the time of this analysis. Overall survival was significantly longer (p=0.0201) in the TCF arm with a HR of 1.29 (95% CI: 1.04–1.61). Efficacy results are summarized in Table 19 and Figures 6 and 7.
Table 19: Efficacy of Docetaxel Injection in the Treatment of Patients with Gastric Adenocarcinoma
| ||
|
Endpoint |
TCF |
CF |
|
Median TTP (months) |
5.6 |
3.7 |
|
(95% CI) |
(4.86–5.91) |
(3.45–4.47) |
|
Hazard ratio* |
0.68 | |
|
(95% CI) |
(0.55–0.84) | |
|
†p-value |
0.0004 | |
|
Median survival (months) |
9.2 |
8.6 |
|
(95% CI) |
(8.38–10.58) |
(7.16–9.46) |
|
Hazard ratio* |
0.77 | |
|
(95% CI) |
(0.62–0.96) | |
|
†p-value |
0.0201 | |
|
Overall Response Rate (CR+PR) (%) |
36.7 |
25.4 |
|
p-value |
0.0106 |
Subgroup analyses were consistent with the overall results across age, gender and race.
|
Figure 6 -Gastric Cancer Study (TAX325) Time to Progression K-M Curve |
|---|
|
|
|
Figure 7 -Gastric Cancer Study (TAX325) Survival K-M Curve |
|---|
|
|
14.6 Head and Neck Cancer
Induction Chemotherapy Followed by Radiotherapy (TAX323)
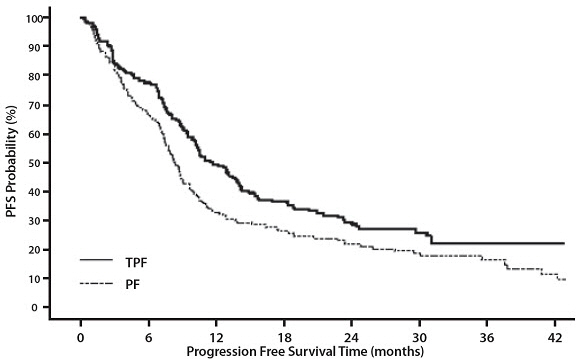
The safety and efficacy of Docetaxel Injection in the induction treatment of patients with squamous cell carcinoma of the head and neck (SCCHN) was evaluated in a multicenter, open-label, randomized trial (TAX323). In this study, 358 patients with inoperable locally advanced SCCHN, and WHO performance status 0 or 1, were randomized to one of two treatment arms. Patients on the Docetaxel Injection arm received Docetaxel Injection (T) 75 mg/m2 followed by cisplatin (P) 75 mg/m2 on Day 1, followed by fluorouracil (F) 750 mg/m2 per day as a continuous infusion on Days 1–5. The cycles were repeated every three weeks for 4 cycles. Patients whose disease did not progress received radiotherapy (RT) according to institutional guidelines (TPF/RT). Patients on the comparator arm received cisplatin (P) 100 mg/m2 on Day 1, followed by fluorouracil (F) 1000 mg/m2/day as a continuous infusion on Days 1–5. The cycles were repeated every three weeks for 4 cycles. Patients whose disease did not progress received RT according to institutional guidelines (PF/RT). At the end of chemotherapy, with a minimal interval of 4 weeks and a maximal interval of 7 weeks, patients whose disease did not progress received radiotherapy (RT) according to institutional guidelines. Locoregional therapy with radiation was delivered either with a conventional fraction regimen (1.8 Gy–2.0 Gy once a day, 5 days per week for a total dose of 66 to 70 Gy) or with an accelerated/hyperfractionated regimen (twice a day, with a minimum interfraction interval of 6 hours, 5 days per week, for a total dose of 70 to 74 Gy, respectively). Surgical resection was allowed following chemotherapy, before or after radiotherapy.
The primary endpoint in this study, progression-free survival (PFS), was significantly longer in the TPF arm compared to the PF arm, p=0.0077 (median PFS: 11.4 vs. 8.3 months, respectively) with an overall median follow-up time of 33.7 months. Median overall survival with a median follow-up of 51.2 months was also significantly longer in favor of the TPF arm compared to the PF arm (median OS: 18.6 vs. 14.2 months, respectively). Efficacy results are presented in Table 20 and Figures 8 and 9.
Table 20: Efficacy of Docetaxel Injection in the Induction Treatment of Patients with Inoperable Locally Advanced SCCHN (Intent-to-Treat Analysis)|
A Hazard ratio of less than 1 favors Docetaxel Injection+cisplatin+fluorouracil | ||
| ||
|
Endpoint |
Docetaxel Injection + Cisplatin + Fluorouracil |
Cisplatin + Fluorouracil |
|
Median progression free survival (months) |
11.4 |
8.3 |
|
(95% CI) |
(10.1–14.0) |
(7.4–9.1) |
|
Adjusted Hazard ratio |
0.71 | |
|
(95% CI) |
(0.56–0.91) | |
|
*p-value |
0.0077 | |
|
Median survival (months) |
18.6 |
14.2 |
|
(95% CI) |
(15.7–24.0) |
(11.5–18.7) |
|
Hazard ratio |
0.71 | |
|
(95% CI) |
(0.56–0.90) | |
|
†p-value |
0.0055 | |
|
Best overall response (CR + PR) to chemotherapy (%) |
67.8 |
53.6 |
|
0.006 | ||
|
Best overall response (CR + PR) to study treatment [chemotherapy +/- radiotherapy] (%) |
72.3 |
58.6 |
|
0.006 |
|
Figure 8 -TAX323 Progression-Free Survival K-M Curve |
|---|
|
|
|
Figure 9 -TAX323 Overall Survival K-M Curve |
|---|
|
|
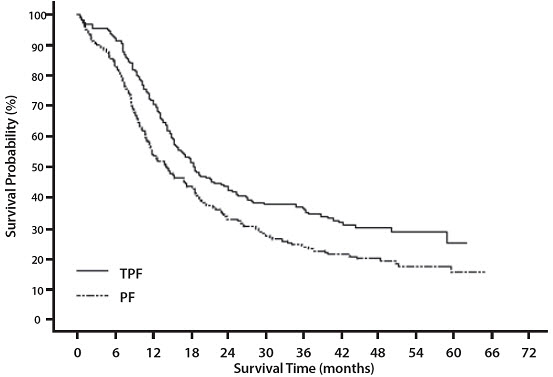
Induction Chemotherapy Followed by Chemoradiotherapy (TAX324)
The safety and efficacy of Docetaxel Injection in the induction treatment of patients with locally advanced (unresectable, low surgical cure, or organ preservation) SCCHN was evaluated in a randomized, multicenter open-label trial (TAX324). In this study, 501 patients, with locally advanced SCCHN, and a WHO performance status of 0 or 1, were randomized to one of two treatment arms. Patients on the Docetaxel Injection arm received Docetaxel Injection (T) 75 mg/m2 by intravenous infusion on day 1 followed by cisplatin (P) 100 mg/m2 administered as a 30-minute to three-hour intravenous infusion, followed by the continuous intravenous infusion of fluorouracil (F) 1000 mg/m2/day from day 1 to day 4. The cycles were repeated every 3 weeks for 3 cycles. Patients on the comparator arm received cisplatin (P) 100 mg/m2 as a 30-minute to three-hour intravenous infusion on day 1 followed by the continuous intravenous infusion of fluorouracil (F) 1000 mg/m2/day from day 1 to day 5. The cycles were repeated every 3 weeks for 3 cycles.
All patients in both treatment arms who did not have progressive disease were to receive 7 weeks of chemoradiotherapy (CRT) following induction chemotherapy 3 to 8 weeks after the start of the last cycle. During radiotherapy, carboplatin (AUC 1.5) was given weekly as a one-hour intravenous infusion for a maximum of 7 doses. Radiation was delivered with megavoltage equipment using once daily fractionation (2 Gy per day, 5 days per week for 7 weeks for a total dose of 70–72 Gy). Surgery on the primary site of disease and/or neck could be considered at anytime following completion of CRT.
The primary efficacy endpoint, overall survival (OS), was significantly longer (log-rank test, p = 0.0058) with the Docetaxel Injection-containing regimen compared to PF [median OS: 70.6 vs. 30.1 months, respectively, hazard ratio (HR) = 0.70, 95% confidence interval (CI) = 0.54–0.90]. Overall survival results are presented in Table 21 and Figure 10.
Table 21: Efficacy of Docetaxel Injection in the Induction Treatment of Patients with Locally Advanced SCCHN (Intent-to-Treat Analysis)|
A Hazard ratio of less than 1 favors Docetaxel Injection+cisplatin+fluorouracil | ||
|
NE - not estimable | ||
| ||
|
Endpoint |
Docetaxel Injection + |
Cisplatin + |
|
Median overall survival (months) |
70.6 |
30.1 |
|
Hazard ratio: |
0.70 |
|
Figure 10 - TAX324 Overall Survival K-M Curve |
|---|
|
|
REFERENCES SECTION
15 REFERENCES
"OSHA Hazardous Drugs." <http://www.osha.gov/SLTC/hazardousdrugs/index.html>
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Bone Marrow Suppression
Advise patients that periodic assessment of their blood count will be performed to detect neutropenia, thrombocytopenia, and/or anemia [see Contraindications (4), Warnings and Precautions (5.3)]. Instruct patients to monitor their temperature frequently and immediately report any occurrence of fever.
Enterocolitis and Neutropenic Colitis
Advise patients of the symptoms of colitis, such as abdominal pain or tenderness, and/or diarrhea, with or without fever, and instruct patients to promptly contact their healthcare provider if they experience these symptoms [see Dosage and Administration (2.7), Warnings and Precautions (5.4)].
Hypersensitivity Reactions
Ask patients whether they have previously received paclitaxel therapy, and if they have experienced a hypersensitivity reaction to paclitaxel. Instruct patients to immediately report to their healthcare provider signs of a hypersensitivity reaction [see Contraindications (4), Warnings and Precautions (5.5)].
Fluid Retention
Advise patients to report signs of fluid retention such as peripheral edema in the lower extremities, weight gain, and dyspnea immediately to their healthcare provider [see Warnings and Precautions (5.6)].
Second Primary Malignancies
Advise patients on the risk of second primary malignancies during treatment with Docetaxel Injection [see Warnings and Precautions (5.7)].
Cutaneous Reactions
Advise patients that localized erythema of the extremities and severe skin toxicities may occur. Instruct patients to immediately report severe cutaneous reactions to their healthcare provider [see Dosage and Administration (2.7), Warnings and Precautions (5.8)].
Neurologic Reactions
Advise patients that neurosensory symptoms or peripheral neuropathy may occur. Instruct patients to immediately report neurologic reactions to their healthcare provider [see Dosage and Administration (2.7), Warnings and Precautions (5.9)].
Eye Disorders
Advise patients that vision disturbances and excessive tearing are associated with Docetaxel Injection administration. Instruct patients to immediately report any vision changes to their healthcare provider [see Warnings and Precautions (5.10)].
Gastrointestinal Reactions
Explain to patients that nausea, vomiting, diarrhea, and constipation are associated with Docetaxel Injection administration. Instruct patients to report any severe events to their healthcare provider [see Adverse Reactions (6)].
Cardiac Disorders
Advise patients to report any irregular and/or rapid heartbeat, severe shortness of breath, dizziness, and/or fainting immediately to their healthcare provider [see Adverse Reactions (6)].
Other Common Adverse Reactions
Advise patients that other common adverse reactions associated with Docetaxel Injection may include alopecia (cases of permanent hair loss have been reported), asthenia, anorexia, dysgeusia, mucositis, myalgia, nail disorders, or pain. Instruct patients to report these reactions to their healthcare provider if serious events occur [see Adverse Reactions (6)].
Importance of Corticosteroids
Explain the significance of oral corticosteroids such as dexamethasone administration to the patient to help facilitate compliance. Instruct patients to report to their healthcare provider if they were not compliant with the oral corticosteroid regimen [see Dosage and Administration (2.6)].
Embryo-Fetal Toxicity
Docetaxel Injection can cause fetal harm. Advise patients to inform their healthcare provider of a known or suspected pregnancy. Advise patients to avoid becoming pregnant while receiving this drug. Advise female patients of reproductive potential to use effective contraceptives during treatment and for 2 months after the last dose of Docetaxel Injection. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of Docetaxel Injection [see Warnings and Precautions (5.12), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during Docetaxel Injection treatment and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males of reproductive potential that Docetaxel Injection may impair fertility [see Nonclinical Toxicology (13.1)].
Alcohol Content in Docetaxel Injection
Explain to patients the possible effects of the alcohol content in Docetaxel Injection, including possible effects on the central nervous system [see Warnings and Precautions (5.13)].
Tumor Lysis Syndrome
Advise patients of the potential risk of tumor lysis syndrome and to immediately report any signs or symptoms associated with this event (nausea, vomiting, confusion, shortness of breath, seizure, irregular heartbeat, dark or cloudy urine, reduced amount of urine, unusual tiredness, muscle cramps) to their healthcare provider. Advise patients of the importance of keeping scheduled appointment for blood work or other laboratory tests and of drinking adequate fluids to avoid dehydration. [see Warnings and Precautions (5.14)].
Ability to Drive or Operate Machines
Explain to patients that Docetaxel Injection may impair their ability to drive or operate machines due to its side effects [see Adverse Reactions (6)] or due to the alcohol content of Docetaxel Injection [see Warnings and Precautions (5.13)]. Advise them not to drive or use machines if they experience these side effects during treatment.
Drug Interactions
Inform patients about the risk of drug interactions and the importance of providing a list of prescription and non-prescription drugs to their healthcare provider [see Drug Interactions (7)].
SPL UNCLASSIFIED SECTION
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA

Novaplus is a registered trademark of Vizient, Inc.
LAB-1495-2.0
SPL PATIENT PACKAGE INSERT SECTION
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: May 2023 | ||
|
Patient Information | |||
|
What is the most important information I should know about Docetaxel
Injection? • o o o • • • Tell your healthcare provider right away if you have any of these signs of a severe allergic reaction: o o o • • o • Tell your healthcare provider right away if you have any of these signs of a severe skin reaction: o o o | |||
|
What is Docetaxel Injection? • • • • • It is not known if Docetaxel Injection is effective in children. | |||
|
Do not receive Docetaxel Injection if you: • • o o See "What is the most important information I should know about Docetaxel
Injection?" for the signs and symptoms of a severe allergic reaction. | |||
|
Before you receive Docetaxel Injection, tell your healthcare provider about all of your medical conditions, including if you: • • • • Females who are able to become pregnant: o o • Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Docetaxel Injection may affect the way other medicines work, and other
medicines may affect the way Docetaxel Injection works. | |||
|
How will I receive Docetaxel Injection? • • • • • | |||
|
What are the possible side effects of Docetaxel Injection? • • • • • | |||
|
o o o o |
o o o o o | ||
|
• The most common side effects of Docetaxel Injection include: | |||
|
• • • • • • • • • |
• • • • • • • • • | ||
|
Tell your healthcare provider if you have a fast or irregular heartbeat,
severe shortness of breath, dizziness or fainting during your infusion. If any
of these events occurs after your infusion, get medical help right away. | |||
|
General information about the safe and effective use of Docetaxel
Injection. | |||
|
What are the ingredients in Docetaxel Injection? Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Novaplus is a registered trademark of Vizient, Inc. LAB-1496-2.0 For more information, call 1-800-441-4100, or go to www.pfizer.com |
|
Every three-week injection of Docetaxel Injection for breast, non-small cell
lung and stomach, and head and neck cancers Oral corticosteroid dosing: (Docetaxel Injection Treatment Day) |
|
Every three-week injection of Docetaxel Injection for prostate cancer Oral corticosteroid dosing: (Docetaxel Injection Treatment Day) |
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with docetaxel have not been performed.
Docetaxel was genotoxic by an aneugenic mechanism in the in vitro chromosome aberration test in CHO-K1 cells and in the in vivo micronucleus test in mice administered doses of 0.39 to 1.56 mg/kg (about 1/60th to 1/15th the recommended human dose on a mg/m2 basis). Docetaxel was not mutagenic in the Ames test or the CHO/HGPRT gene mutation assays.
Docetaxel did not reduce fertility in rats when administered in multiple intravenous doses of up to 0.3 mg/kg (about 1/50th the recommended human dose on a mg/m2 basis), but decreased testicular weights were reported. This correlates with findings of a 10-cycle toxicity study (dosing once every 21 days for 6 months) in rats and dogs in which testicular atrophy or degeneration was observed at intravenous doses of 5 mg/kg in rats and 0.375 mg/kg in dogs (about 1/3rd and 1/15th the recommended human dose on a mg/m2 basis, respectively). An increased frequency of dosing in rats produced similar effects at lower dose levels.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Docetaxel Injection, USP is supplied in single-dose or multiple-dose vials as a sterile, pyrogen-free, non-aqueous colorless to pale yellow solution. Discard unused portion of the single-dose vial.
The following strengths are available in a one-vial formulation:
|
Unit of Sale |
Concentration |
|
NDC 0409-2026-01 |
20 mg/2 mL |
|
NDC 0409-0016-01 |
160 mg/16 mL |
16.2 Storage
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature]. Retain in the original package to protect from light. Freezing does not adversely affect the product.
After first use and following multiple needle entries and product withdrawals, Docetaxel Injection multiple-dose vials are stable for up to 28 days when stored between 2°C and 8°C (36°F and 46°F) and protected from light.
16.3 Handling and Disposal
Docetaxel Injection is a hazardous drug. Follow applicable special handling and disposal procedures.1