Omeprazole
Omeprazole DR 20mg Capsules
af85afda-6b0c-d657-e053-2a95a90afb4b
HUMAN PRESCRIPTION DRUG LABEL
Apr 17, 2023
Advanced Rx Pharmacy of Tennessee, LLC
DUNS: 117023142
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Omeprazole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel




DESCRIPTION SECTION
11. Description
The active ingredient in Omeprazole Delayed-Release Capsules, USP is a substituted benzimidazole, 5-methoxy-2[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42 g/mol. The structural formula is:
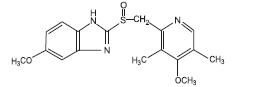
Omeprazole, USP is a white to off-white crystalline powder that melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
Omeprazole, USP is supplied as delayed-release capsules for oral administration. Each delayed-release capsule contains either 10 mg, 20 mg or 40 mg of omeprazole, USP in the form of enteric-coated granules with the following inactive ingredients: anhydrous lactose, cetyl alcohol, di-sodium hydrogen phosphate dihydrate, hypromellose, hypromellose phthalate, mannitol, simethicone emulsion 30%, sodium lauryl sulfate and sugar sphere.
The capsule shell for Omeprazole Delayed-Release Capsules, USP 10 mg contains D&C Yellow No.10, FD&C Blue No.1, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, sodium lauryl sulfate and titanium dioxide.
The capsule shell for Omeprazole Delayed-Release Capsules, USP 20 mg contains FD&C Blue No.1, gelatin, sodium lauryl sulfate and titanium dioxide.
The capsule shell for Omeprazole Delayed-Release Capsules, USP 40 mg contains D&C Yellow No.10, FD&C Blue No.1, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, sodium lauryl sulfate and titanium dioxide.
The imprinting ink has the following components: black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, strong ammonia solution and shellac.
