Arnica Montana
3c62d081-4178-4ac0-b81f-d97202dee8ea
HUMAN OTC DRUG LABEL
Aug 13, 2025
NARTEX LABORATORIOS HOMEOPATICOS SA DE CV
DUNS: 589914576
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Arnica Montana
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
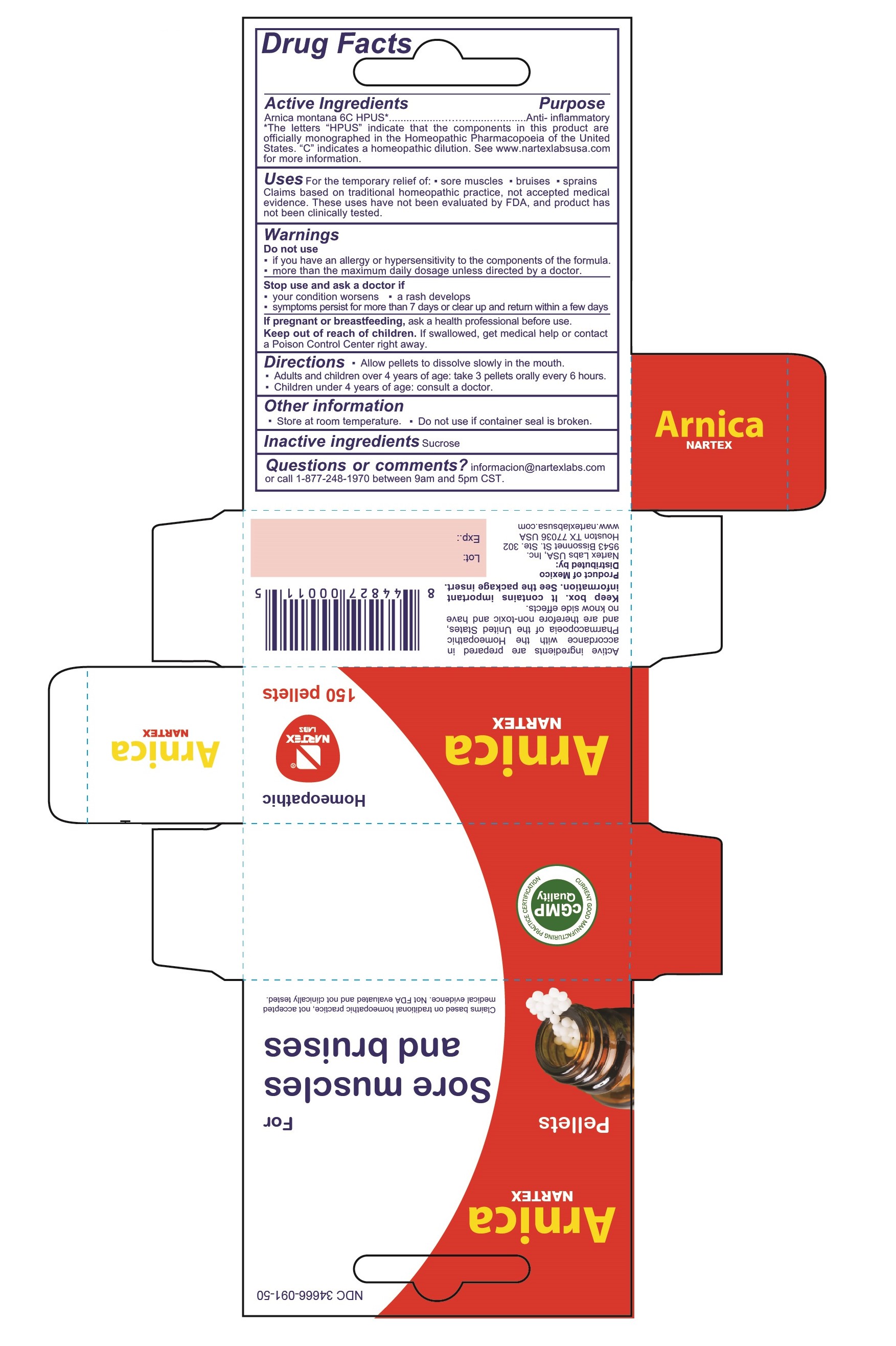
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 34666-091-50
Arnica
Nartex
Pellets
For Sore muscles and bruises
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated and not clinically tested.
Homeopathic
150 pellets

INDICATIONS & USAGE SECTION
For the temporary relief of:
- sore muscles
- bruises
- sprains
Claims based on traditional homeopathic practice, not accepted medical evidence. These uses have not been evaluated by FDA, and product has not been clinically tested.
OTC - ACTIVE INGREDIENT SECTION
Arnica montana......6C HPUS
OTC - PURPOSE SECTION
Arnica montana.......6C HPUS*......anti-inflammatory
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. "C" indicates a homeopathic dilution. See www.nartexlabsusa.com for more information.
WARNINGS SECTION
Do not use
- if you have an allergy or hypersensitivity to the components of the formula.
- more than the maximum daily dosage unless directed by a doctor.
OTC - STOP USE SECTION
Stop use and ask a doctor if:
- condition worsens
- a rash develops
- symptoms persist for more than 7 days or clear up and return within a few days
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast feeding, consult a health professional.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
**Keep out of reach of children.**If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- Allow pellets to dissolve slowly in the mouth.
- Adults and children over 4 years of age: take 3 pellets orally every 6 hours.
- Children under 4 years of age: consult a doctor.
STORAGE AND HANDLING SECTION
- Store at room temperature.
- Do not use if container seal is broken.
INACTIVE INGREDIENT SECTION
Sucrose
OTC - QUESTIONS SECTION
Questions or comments?informacion@nartexlabs.comor call 1-877-248-1970 between 9am and 5pm CST
DESCRIPTION SECTION
Distributed by:
Nartex Labs USA, Inc.
9543 Bissonnet St. Ste. 302
Houston TX 77036 USA
www.nartexlabsusa.com
