Niva Thyroid
Niva Thyroid (thyroid tablets, USP) Rx only
eae7789a-d85d-4b04-a680-e688a371ebee
HUMAN PRESCRIPTION DRUG LABEL
Nov 2, 2023
Nivagen Pharmaceuticals, Inc.
DUNS: 052032418
Products 8
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
thyroid, porcine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 75834-310-01
Niva Thyroid (Thyroid Tablets, USP)
** 1/4 GRAIN (15 mg)**
Each tablet contains:
levothyroxine (T4) 9.5 mcg
liothyronine (T3) 2.25 mcg
100 TABLETS

NDC 75834-311-01
Niva Thyroid (Thyroid Tablets, USP)
** 1/2 GRAIN (30 mg)**
Each tablet contains:
levothyroxine (T4) 19 mcg
liothyronine (T3) 4.5 mcg
100 TABLETS

NDC 75834-312-01
Niva Thyroid (Thyroid Tablets, USP)
** 1 GRAIN (60 mg)**
Each tablet contains:
levothyroxine (T4) 38 mcg
liothyronine (T3) 9 mcg
100 TABLETS

NDC 75834-313-01
Niva Thyroid (Thyroid Tablets, USP)
** 1 ½ GRAIN (90 mg)**
Each tablet contains:
levothyroxine (T4) 57 mcg
liothyronine (T3) 13.5 mcg
100 TABLETS

NDC 75834-314-01
Niva Thyroid (Thyroid Tablets, USP)
** 2 GRAIN (120 mg)**
Each tablet contains:
levothyroxine (T4) 76 mcg
liothyronine (T3) 18 mcg
100 TABLETS

NDC 75834-315-01
Niva Thyroid (Thyroid Tablets, USP)
** 3 GRAIN (180 mg)**
Each tablet contains:
levothyroxine (T4) 114 mcg
liothyronine (T3) 27 mcg
100 TABLETS

NDC 75834-316-01
Niva Thyroid (Thyroid Tablets, USP)
** 4 GRAIN (240 mg)**
Each tablet contains:
levothyroxine (T4) 152 mcg
liothyronine (T3) 36 mcg
100 TABLETS

NDC 75834-317-01
Niva Thyroid (Thyroid Tablets, USP)
** 5 GRAIN (300 mg)**
Each tablet contains:
levothyroxine (T4) 190 mcg
liothyronine (T3) 45 mcg
100 TABLETS

DESCRIPTION SECTION
DESCRIPTION
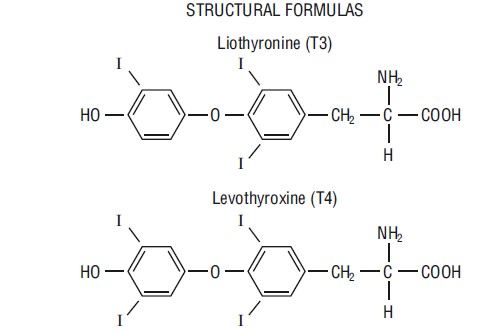
Niva Thyroid (thyroid tablets, USP)* for oral use is a natural preparation derived from porcine thyroid glands and has a strong, characteristic odor. (T3 liothyronine is approximately four times as potent as T4 levothyroxine on a microgram for microgram basis.) They provide 38 mcg levothyroxine (T4) and 9 mcg liothyronine (T3) per grain of thyroid. The inactive ingredients are microcrystalline cellulose, sodium starch glycolate, povidone, and calcium stearate.