Choleystyramine Light
Cholestyramine for Oral Suspension USP, Light Rx only
4c9d4561-d5b5-463b-ac57-1bc4d84d135d
HUMAN PRESCRIPTION DRUG LABEL
Jul 7, 2022
Ascend Laboratories, LLC
DUNS: 141250469
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Choleystyramine Light
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
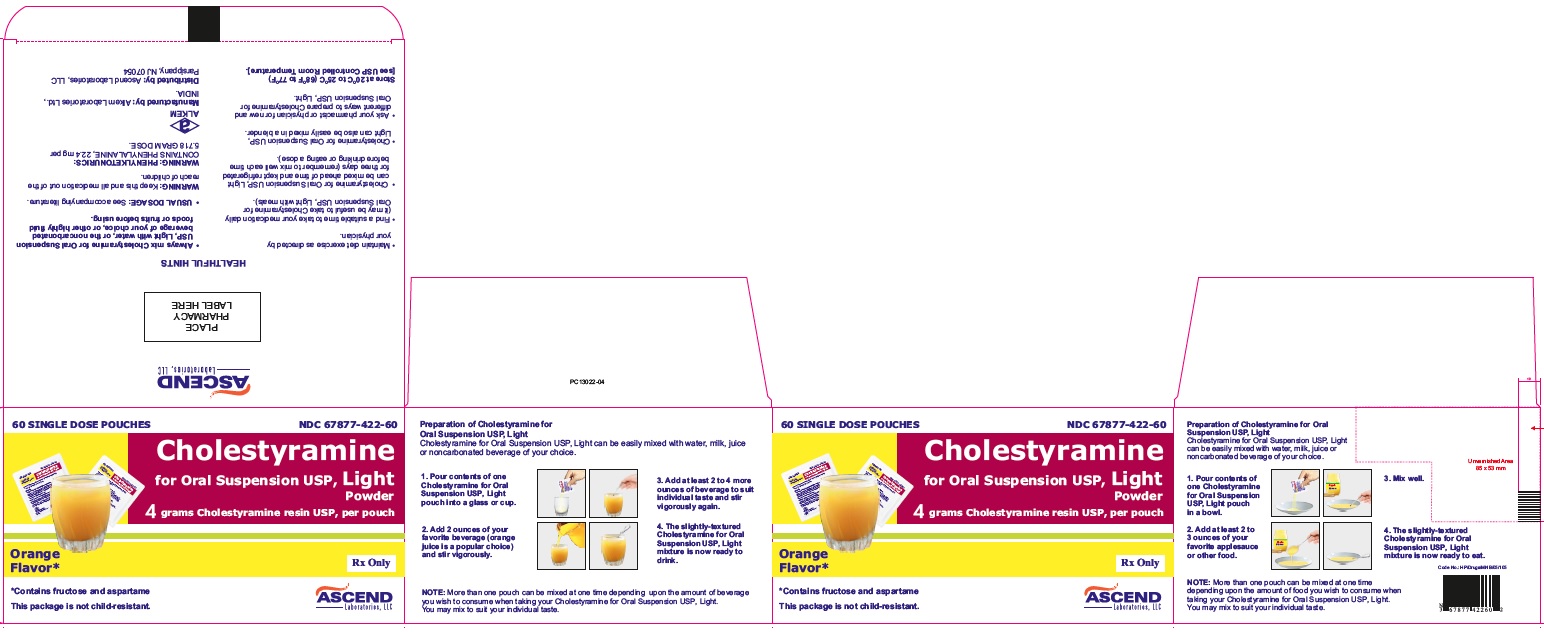
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 67877-422-24
Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per scoopful.
Orange Flavor

NDC 67877-422-60
Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per pouch.
Orange Flavor
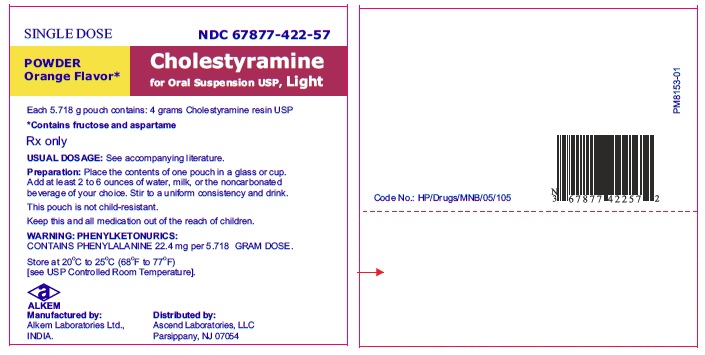
NDC 67877-422-57
Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per pouch.
Orange Flavor
Indications & Usage Section
INDICATIONS AND USAGE
- Cholestyramine for Oral Suspension USP Light powder is indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low density lipoprotein [LDL] cholesterol) who do not respond adequately to diet. Cholestyramine for Oral Suspension USP Light powder may be useful to lower LDL cholesterol in patients who also have hypertriglyceridemia, but it is not indicated where hypertriglyceridemia is the abnormality of most concern.
Therapy with lipid-altering agents should be a component of multiple risk
factor intervention in those individuals at significantly increased risk for
atherosclerotic vascular disease due to
hypercholesterolemia. Treatment should begin and continue with dietary therapy
specific for the type of hyperlipoproteinemia determined prior to initiation
of drug therapy. Excess body weight may be an important factor and caloric
restriction for weight normalization should be addressed prior to drug therapy
in the overweight.
Prior to initiating therapy with cholestyramine resin, secondary causes of hypercholesterolemia (e.g., poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, alcoholism), should be excluded and a lipid profile performed to assess Total cholesterol, HDL-C and triglycerides (TG). For individuals with TG less than 400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation:
LDL-C = Total cholesterol - [(TG/5) + HDL-C]
For TG levels > 400 mg/dL, this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation. In hypertriglyceridemic patients, LDL-C may be low or normal despite elevated Total-C. In such cases cholestyramine resin may not be indicated.
Serum cholesterol and triglyceride levels should be determined periodically
based on NCEP guidelines to confirm initial and adequate long-term response. A
favorable trend in cholesterol
reduction should occur during the first month of cholestyramine resin therapy.
The therapy should be continued to sustain cholesterol reduction. If adequate
cholesterol reduction is not attained, increasing the dosage of cholestyramine
resin or adding other lipid-lowering agents in combination with cholestyramine
resin should be considered.
Since the goal of treatment is to lower LDL-C, the NCEP4 recommends that LDL-C levels be used to initiate and assess treatment response. If LDL-C levels are not available then Total-C alone may be used to monitor long-term therapy. A lipoprotein analysis (including LDL-C determination) should be carried out once a year. The NCEP treatment guidelines are summarized below.
| |||
|
LDL-Cholesterol mg/dL (mmol/L) | |||
|
Definite Atherosclerotic Disease* |
Two or More Other |
Initiation Level |
Goal |
|
No |
No |
≥190 (≥4.9) |
<160 (<4.1) |
|
No |
Yes |
≥160 (≥4.1) |
<130 (<3.4) |
|
Yes |
Yes or No |
≥130 (≥3.4) |
≤100 (≤2.6) |
Cholestyramine resin monotherapy has been demonstrated to retard the rate of progression2,3 and increase the rate of regression3 of coronary atherosclerosis.
- Cholestyramine for Oral Suspension USP Light powder, is indicated for the relief of pruritus associated with partial biliary obstruction. Cholestyramine resin has been shown to have a variable effect on serum cholesterol in these patients. Patients with primary biliary cirrhosis may exhibit an elevated cholesterol as part of their disease.
Contraindications Section
CONTRAINDICATIONS
Cholestyramine for Oral Suspension USP Light powder is contraindicated in patients with complete biliary obstruction where bile is not secreted into the intestine and in those individuals who have shown hypersensitivity to any of its components.
Adverse Reactions Section
ADVERSE REACTIONS
The most common adverse reaction is constipation. When used as a cholesterol- lowering agent predisposing factors for most complaints of constipation are high dose and increased age (more than 60 years old). Most instances of constipation are mild, transient and controlled with conventional therapy.
Some patients require a temporary decrease in dosage or discontinuation of
therapy.
Less Frequent Adverse Reactions - Abdominal discomfort and/or pain,
flatulence, nausea, vomiting, diarrhea, eructation, anorexia, steatorrhea,
bleeding tendencies due to hypoprothrombinemia (Vitamin K deficiency) as well
as Vitamin A (one case of night blindness reported) and D deficiencies,
hyperchloremic acidosis in children, osteoporosis, rash and irritation of the
skin, tongue and perianal area. Rare reports of intestinal obstruction,
including two deaths, have been reported in pediatric patients.
Occasional calcified material has been observed in the biliary tree, including calcification of the gallbladder, in patients to whom cholestyramine resin has been given. However, this may be a manifestation of the liver disease and not drug related.
One patient experienced biliary colic on each of three occasions on which he took a cholestyramine for oral suspension product. One patient diagnosed as acute abdominal symptom complex was found to have a “pasty mass” in the transverse colon on x-ray.
Other events (not necessarily drug related) reported in patients taking cholestyramine resin include:
Gastrointestinal: GI-rectal bleeding, black stools, hemorrhoidal bleeding, bleeding from known duodenal ulcer, dysphagia, hiccups, ulcer attack, sour taste, pancreatitis, rectal pain, diverticulitis.
Laboratory Test Changes: Liver function abnormalities.
Hematologic: Prolonged prothrombin time, ecchymosis, anemia.
Hypersensitivity: Urticaria, asthma, wheezing, shortness of breath.
Musculoskeletal: Backache, muscle and joint pains, arthritis.
Neurologic: Headache, anxiety, vertigo, dizziness, fatigue, tinnitus, syncope, drowsiness, femoral nerve pain, paresthesia.
Eye: Uveitis.
Renal: Hematuria, dysuria, burnt odor to urine, diuresis.
Miscellaneous: Weight loss, weight gain, increased libido, swollen glands, edema, dental bleeding, dental caries, erosion of tooth enamel, tooth discoloration.
Clinical Pharmacology Section
CLINICAL PHARMACOLOGY
Cholesterol is probably the sole precursor of bile acids. During normal digestion, bile acids are secreted into the intestines. A major portion of the bile acids is absorbed from the intestinal tract and returned to the liver via the enterohepatic circulation. Only very small amounts of bile acids are found in normal serum.
Cholestyramine resin adsorbs and combines with the bile acids in the intestine to form an insoluble complex which is excreted in the feces. This results in a partial removal of bile acids from the enterohepatic circulation by preventing their absorption.
The increased fecal loss of bile acids due to cholestyramine resin administration leads to an increased oxidation of cholesterol to bile acids, a decrease in beta lipoprotein or low density lipoprotein plasma levels and a decrease in serum cholesterol levels. Although in man, cholestyramine resin produces an increase in hepatic synthesis of cholesterol, plasma cholesterol levels fall.
In patients with partial biliary obstruction, the reduction of serum bile acid levels by cholestyramine resin reduces excess bile acids deposited in the dermal tissue with resultant decrease in pruritus.
Clinical Studies
In a large, placebo-controlled, multi-clinic study, LRC-CPPT1 ,
hypercholesterolemic subjects treated with cholestyramine resin had mean
reductions in total and low-density lipoprotein cholesterol (LDL-C) which
exceeded those for diet and placebo treatment by 7.2% and 10.4%, respectively.
Over the seven year study period the cholestyramine resin group experienced a
19% reduction (relative to the incidence in the placebo group) in the combined
rate of coronary heart disease death plus non-fatal myocardial infarction
(cumulative incidences of 7% cholestyramine resin and 8.6% placebo). The
subjects included in the study were men aged 35 to 59 with serum cholesterol
levels above 265 mg/dL and no previous history of heart disease. It is not
clear to what extent these findings can be extrapolated to females and other
segments of the hypercholesterolemic population. (See also
PRECAUTIONS**,Carcinogenesis, Mutagenesis, Impairment of
Fertility.**)
Two controlled clinical trials have examined the effects of cholestyramine monotherapy upon coronary atherosclerotic lesions using coronary arteriography. In the NHLBI Type II Coronary Intervention Trial2, 116 patients (80% male) with coronary artery disease (CAD) documented by arteriography were randomized to cholestyramine resin or placebo for five years of treatment. Final study arteriography revealed progression of coronary artery disease in 49% of placebo patients compared to 32% of the cholestyramine resin group (p<0.05).
In the St. Thomas Atherosclerosis Regression Study (STARS)3, 90 hypercholesterolemic men with CAD were randomized to three blinded treatments: usual care, lipid-lowering diet and lipid-lowering diet plus cholestyramine resin. After 36 months, follow-up coronary arteriography revealed progression of disease in 46% of usual care patients, 15% of patients on lipid-lowering diet and 12% of those receiving diet plus cholestyramine resin (p<0.02). The mean absolute width of coronary segments decreased in the usual care group, increased slightly (0.003 mm) in the diet group and increased by 0.103 mm in the diet plus cholestyramine group (p<0.05). Thus in these randomized controlled clinical trials using coronary arteriography, cholestyramine resin monotherapy has been demonstrated to slow progression2,3 and promote regression3 of atherosclerotic lesions in the coronary arteries of patients with coronary artery disease.
The effect of intensive lipid-lowering therapy on coronary atherosclerosis has been assessed by arteriography in hyperlipidemic patients. In these randomized, controlled clinical trials, patients were treated for two to four years by either conventional methods (diet, placebo or in some cases low dose resin) or intensive combination therapy using diet plus colestipol (an anion exchange resin with a mechanism of action and an effect similar on serum lipids to that of Cholestyramine for Oral Suspension Light) plus either nicotinic acid or lovastatin. When compared to conventional measures, intensive lipid-lowering combination therapy significantly reduced the frequency of progression and increased the frequency of regression of coronary atherosclerotic lesions in patients with or at risk for coronary artery disease.
Description Section
DESCRIPTION
Cholestyramine for Oral Suspension USP Light powder, the chloride salt of a basic anion exchange resin, a cholesterol-lowering agent, is intended for oral administration. Cholestyramine resin is quite hydrophilic, but insoluble in water. Cholestyramine resin is not absorbed from the digestive tract. Each 5.718 grams of Cholestyramine for Oral Suspension USP Light powder contain 4 grams of cholestyramine resin. It is represented by the following structural formula:
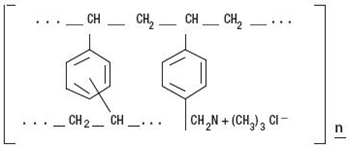
** Representation of structure of main polymeric groups**
Inactive Ingredients: mannitol, fructose, sorbitol, aspartame, citric acid, lake pigment 6010 D&C yellow #10 aluminum lake and FD&C yellow #6/sunset yellow FCF AI 15% - 18%, orange flavour, propylene glycol alginate, xanthan gum, pectin, silicon dioxide.****
Warnings Section
WARNINGS
PHENYLKETONURICS:
** CHOLESTYRAMINE FOR ORAL SUSPENSION USP LIGHT POWDER CONTAINS 22.4 mg
PHENYLALANINE PER 5.718 GRAM DOSE.**
Precautions Section
PRECAUTIONS
General
Chronic use of cholestyramine resin may be associated with increased bleeding tendency due to hypoprothrombinemia associated with Vitamin K deficiency. This will usually respond promptly to parenteral Vitamin K1 and recurrences can be prevented by oral administration of Vitamin K1. Reduction of serum or red cell folate has been reported over long term administration of cholestyramine resin. Supplementation with folic acid should be considered in these cases.
There is a possibility that prolonged use of cholestyramine resin, since it is a chloride form of anion exchange resin, may produce hyperchloremic acidosis. This would especially be true in younger and smaller patients where the relative dosage may be higher. Caution should also be exercised in patients with renal insufficiency or volume depletion and in patients receiving concomitant spironolactone. Cholestyramine resin may produce or worsen preexisting constipation. The dosage should be increased gradually in patients to minimize the risk of developing fecal impaction. In patients with pre- existing constipation, the starting dose should be 1 pouch or 1 scoop once daily for 5 to 7 days, increasing to twice daily with monitoring of constipation and of serum lipoproteins, at least twice, 4 to 6 weeks apart. Increased fluid intake and fiber intake should be encouraged to alleviate constipation and a stool softener may occasionally be indicated. If the initial dose is well tolerated, the dose may be increased as needed by one dose/day (at monthly intervals) with periodic monitoring of serum lipoproteins. If constipation worsens or the desired therapeutic response is not achieved at one to six doses/day, combination therapy or alternate therapy should be considered. Particular effort should be made to avoid constipation in patients with symptomatic coronary artery disease. Constipation associated with cholestyramine resin may aggravate hemorrhoids.
Information for Patients
Inform your physician if you are pregnant or plan to become pregnant or are breast-feeding. Drink plenty of fluids and mix each 5.7 gram dose of Cholestyramine for Oral Suspension USP Light powder in at least 2 to 6 ounces of fluid before taking. Sipping or holding the resin suspension in the mouth for prolonged periods may lead to changes in the surface of the teeth resulting in discoloration, erosion of enamel or decay; good oral hygiene should be maintained.
Laboratory Tests
Serum cholesterol levels should be determined frequently during the first few
months of therapy and periodically thereafter. Serum triglyceride levels
should be measured periodically to detect whether significant changes have
occurred.
The LRC-CPPT showed a dose-related increase in serum triglycerides of 10.7% to
17.1% in the cholestyramine-treated group, compared with an increase of 7.9%
to 11.7% in the placebo group. Based on the mean values and adjusting for the
placebo group, the cholestyramine-treated group showed an increase of 5% over
pre-entry levels the first year of the study and an increase of 4.3% the
seventh year.
Drug Interactions
Cholestyramine resin may delay or reduce the absorption of concomitant oral medication such as phenylbutazone, warfarin, thiazide diuretics (acidic) or propranolol (basic), as well as tetracycline, penicillin G, phenobarbital, thyroid and thyroxine preparations, estrogens and progestins and digitalis.Interference with the absorption of oral phosphate supplements has been observed with another positively-charged bile acid sequestrant. Cholestyramine resin may interfere with the pharmacokinetics of drugs that undergo enterohepatic circulation. The discontinuance of cholestyramine resin could pose a hazard to health if a potentially toxic drug such as digitalis has been titrated to a maintenance level while the patient was taking cholestyramine resin.
Because cholestyramine binds bile acids, cholestyramine resin may interfere with normal fat digestion and absorption and thus may prevent absorption of fat-soluble vitamins such as A, D, E and K. When cholestyramine resin is given for long periods of time, concomitant supplementation with water miscible (or parenteral) forms of fat soluble vitamins should be considered.
SINCE CHOLESTYRAMINE RESIN MAY BIND OTHER DRUGS GIVEN CONCURRENTLY, IT IS RECOMMENDED THAT PATIENTS TAKE OTHER DRUGS AT LEAST 1 HOUR BEFORE OR 4 TO 6 HOURS AFTER CHOLESTYRAMINE RESIN (OR AT AS GREAT AN INTERVAL AS POSSIBLE) TO AVOID IMPEDING THEIR ABSORPTION.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In studies conducted in rats in which cholestyramine resin was used as a tool to investigate the role of various intestinal factors, such as fat, bile salts and microbial flora, in the development of intestinal tumors induced by potent carcinogens, the incidence of such tumors was observed to be greater in cholestyramine resin-treated rats than in control rats.
The relevance of this laboratory observation from studies in rats to the clinical use of cholestyramine resin is not known. In the LRC-CPPT study referred to above, the total incidence of fatal and nonfatal neoplasms was similar in both treatment groups. When the many different categories of tumors are examined, various alimentary system cancers were somewhat more prevalent in the cholestyramine group. The small numbers and the multiple categories prevent conclusions from being drawn. However, in view of the fact that cholestyramine resin is confined to the GI tract and not absorbed and in light of the animal experiments referred to above, a six-year post-trial follow-up of the LRC-CPPT5 patient population has been completed (a total of 13.4 years of in-trial plus post-trial follow-up) and revealed no significant difference in the incidence of cause-specific mortality or cancer morbidity between cholestyramine and placebo treated patients.
Pregnancy
There are no adequate and well controlled studies in pregnant women. The use of cholestyramine in pregnancy or lactation or by women of childbearing age requires that the potential benefits of drug therapy be weighted against the possible hazards to the mother and child. Cholestyramine is not absorbed systemically, however, it is known to interfere with absorption of fat-soluble vitamins; accordingly, regular prenatal supplementation may not be adequate (see**PRECAUTIONS****,**Drug Interactions).
Nursing Mothers
Caution should be exercised when cholestyramine resin is administered to a nursing mother. The possible lack of proper vitamin absorption described in PRECAUTIONS**,****Pregnancy**may have an effect on nursing infants.
Pediatric Use
Although an optimal dosage schedule has not been established, standard texts(6,7) list a usual pediatric dose of 240 mg/kg/day of anhydrous cholestyramine resin in two to three divided doses, normally not to exceed 8 g/day with dose titration based on response and tolerance.
In calculating pediatric dosages, 70.2 mg of anhydrous cholestyramine resin are contained in 100.35 mg of Cholestyramine for Oral Suspension USP Light.
The effects of long-term drug administration, as well as its effect in maintaining lowered cholesterol levels in pediatric patients, are unknown. Also seeADVERSE REACTIONS.
Overdosage Section
OVERDOSAGE
Overdosage of cholestyramine resin has been reported in a patient taking 150% of the maximum recommended daily dosage for a period of several weeks. No ill effects were reported. Should an overdosage occur, the chief potential harm would be obstruction of the gastrointestinal tract. The location of such potential obstruction, the degree of obstruction and the presence or absence of normal gut motility would determine treatment.
Dosage & Administration Section
DOSAGE AND ADMINISTRATION
The recommended starting adult dose for Cholestyramine for Oral Suspension USP Light powder is one pouch or one level scoopful (5.718 grams of Cholestyramine for Oral Suspension USP Light powder contains 4 grams of cholestyramine resin) once or twice a day. The recommended maintenance dose for Cholestyramine for Oral Suspension USP Light powder is 2 to 4 pouches or scoopfuls daily (8 to 16 grams anhydrous cholestyramine resin) divided into two doses. It is recommended that increases in dose be gradual with periodic assessment of lipid/lipoprotein levels at intervals of not less than 4 weeks. The maximum recommended daily dose is 6 pouches or scoopfuls of Cholestyramine for Oral Suspension USP Light powder (24 grams of anhydrous cholestyramine resin).
The suggested time of administration is at mealtime but may be modified to avoid interference with absorption of other medications. Although the recommended dosing schedule is twice daily, Cholestyramine for Oral Suspension USP Light powder may be administered in 1 to 6 doses per day.
Cholestyramine for Oral Suspension USP Light powder should not be taken in its dry form. Always mix the dry powder with water or other fluids before ingesting. See Preparation Instructions.
Concomitant Therapy
Preliminary evidence suggests that the lipid-lowering effects of cholestyramine on total and LDL-cholesterol are enhanced when combined with a HMG-CoA reductase inhibitor, e.g., pravastatin, lovastatin, simvastatin and fluvastatin. Additive effects on LDL-cholesterol are also seen with combined nicotinic acid/cholestyramine therapy. See the**PRECAUTIONS****,**Drug Interactions for recommendations on administering concomitant therapy.
Preparation
The color of Cholestyramine for Oral Suspension USP Light powder may vary
somewhat from batch to batch but this variation does not affect the
performance of the product. Place the contents of one single-dose pouch or one
level scoopful of Cholestyramine for Oral Suspension USP Light powder in a
glass or cup. Add at least 2 to 6 ounces of water or other noncarbonated
beverage of your choice. Stir to a uniform consistency and drink.
Cholestyramine for Oral Suspension USP Light powder may also be mixed with
highly fluid soups or pulpy fruits with a high moisture content such as
applesauce or crushed pineapple.
How Supplied Section
HOW SUPPLIED
Cholestyramine for Oral Suspension USP Light powder orange flavor is available in cartons of sixty 5.718 gram pouches and in jars containing 240.156 grams. Each 5.718 gram dose of Cholestyramine for Oral Suspension USP Light powder contains 4 grams of anhydrous cholestyramine resin.
Pouch: Pale yellow to yellow powder filled in sealed paper foil pouch.
67877-422-60
Carton of 60 pouches
67877-422-57
5.718 gram pouch
Jar: Pale yellow to yellow powder filled in sealed white HDPE jar.
67877-422-24
240.156 gram (Jar Pack) (containing a scoop that is not interchangeable with
scoops from other products)
Storage
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
REFERENCES
1. The Lipid Research Clinics Coronary Primary Prevention Trial Results: (I)
Reduction in Incidence of Coronary Heart Disease; (II) The Relationship of
Reduction in Incidence of Coronary Heart Disease to Cholesterol Lowering.
JAMA. 1984; 251:351-374.
2. Brensike JF, Levy RI, Kelsey SF, et al. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI type II coronary intervention study. Circulation 1984; 69:313-24.
3. Watte, GF, Lewis B, Brunt JNH, Lewis ES, et al. Effects on coronary artery disease of lipid-lowering diet or diet plus cholestyramine, in the St. Thomas Atherosclerosis Regression Study (STARS). Lancet 1992; 339:563-69.
4. National Cholesterol Education Program. Second Report of the Expert panel on Detection,Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation 1994 Mar;89 (3):1333-445.
5. The Lipid Research Clinics Investigators. The Lipid Research Clinics Coronary Primary Prevention Trial: Results of 6 Years of Post-Trial Follow-up. Arch Intern Med 1992; 152:1399-1410.
6. Behrman RE et al (eds): Nelson, Textbook of Pediatrics, ed 15. Philadelphia, PA, WB Saunders Company, 1996.
7. Takemoto CK et al (eds): Pediatric Dosage Handbook, ed 3. Cleveland/Akron, OH, Lexi-Comp, Inc., 1996/1997.
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
Alkem Laboratories Ltd.,
INDIA.
Distributed by:
****Ascend Laboratories, LLC
339 Jefferson Road,
Parsippany, NJ 07054
**Revised:**November 2021
PT9076-02
