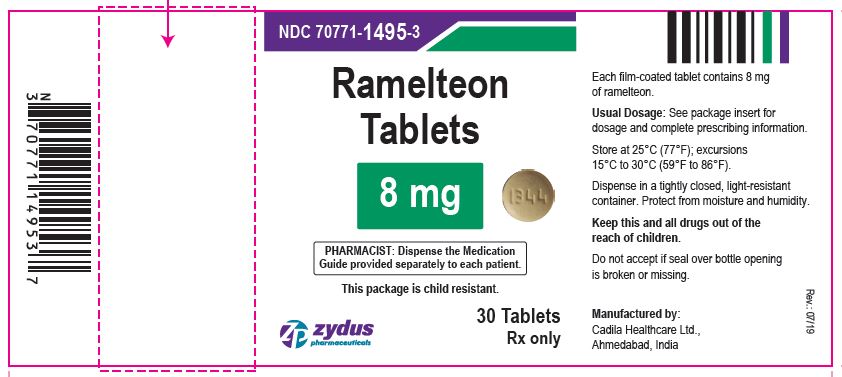
Ramelteon
RAMELTEON TABLETS
Approved
Approval ID
14ba7994-476a-4978-8cac-29989335a63c
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Sep 27, 2023
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ramelteon
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1495
Application NumberANDA211567
Product Classification
M
Marketing Category
C73584
G
Generic Name
Ramelteon
Product Specifications
Route of AdministrationORAL
Effective DateSeptember 27, 2023
FDA Product Classification
INGREDIENTS (12)
FERROSOFERRIC OXIDEInactive
Code: XM0M87F357
Classification: IACT
RAMELTEONActive
Quantity: 8 mg in 1 1
Code: 901AS54I69
Classification: ACTIB
ANHYDROUS LACTOSEInactive
Code: 3SY5LH9PMK
Classification: IACT
FERRIC OXIDE YELLOWInactive
Code: EX438O2MRT
Classification: IACT
FERRIC OXIDE REDInactive
Code: 1K09F3G675
Classification: IACT
HYPROMELLOSE 2910 (3 MPA.S)Inactive
Code: 0VUT3PMY82
Classification: IACT
POLYETHYLENE GLYCOL 6000Inactive
Code: 30IQX730WE
Classification: IACT
POVIDONEInactive
Code: FZ989GH94E
Classification: IACT
STARCH, CORNInactive
Code: O8232NY3SJ
Classification: IACT
SILICON DIOXIDEInactive
Code: ETJ7Z6XBU4
Classification: IACT
SODIUM STEARYL FUMARATEInactive
Code: 7CV7WJK4UI
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 9/27/2023
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1495-3
Ramelteon Tablets, 8 mg
30 Tablets
Rx only