DESIPRAMINE HYDROCHLORIDE
Desipramine Hydrochloride Tablets, USP
ee14dd2c-c54b-4cd1-a3a0-f7ad5a5a0ed0
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
DUNS: 780779901
Products 6
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
desipramine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 23155-578-01
Desipramine Hydrochloride Tablets, USP
10 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
100 Tablets
Rx only
Avet Pharma Ltd.

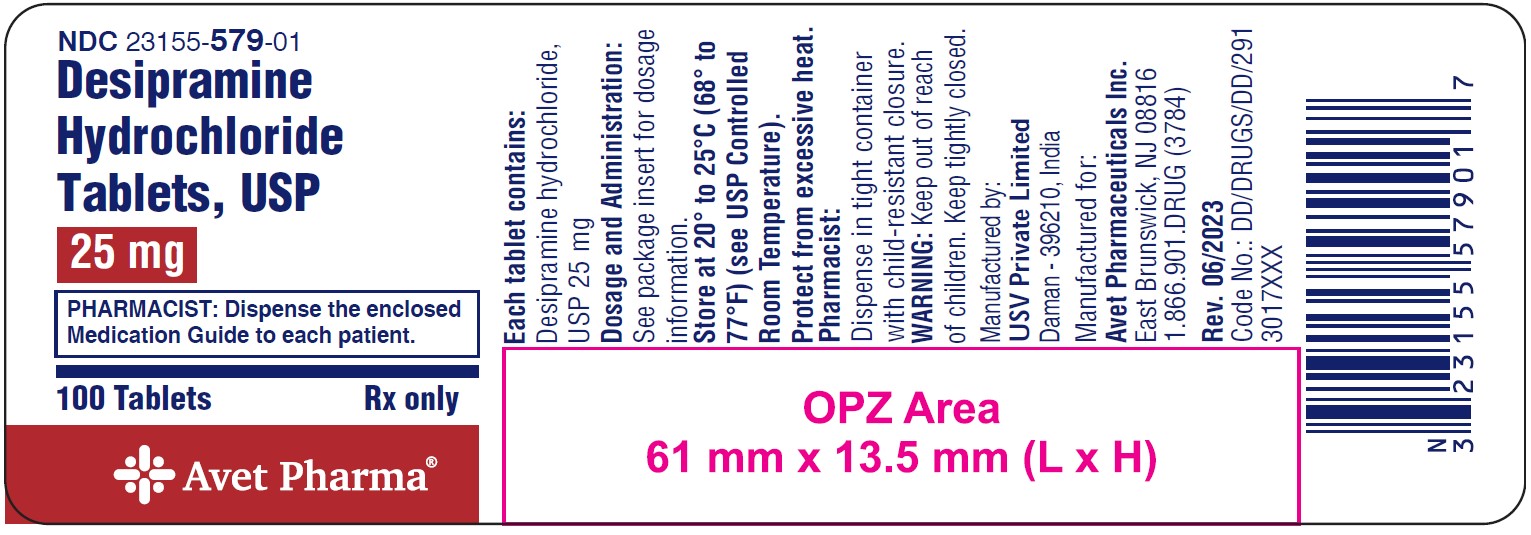
NDC 23155-579-01
Desipramine Hydrochloride Tablets, USP
25 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
100 Tablets
Rx only
Avet Pharma Ltd.
NDC 23155-580-01
Desipramine Hydrochloride Tablets, USP
50 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
100 Tablets
Rx only
Avet Pharma Ltd.

NDC 23155-581-01
Desipramine Hydrochloride Tablets, USP
75 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
100 Tablets
Rx only
Avet Pharma Ltd.

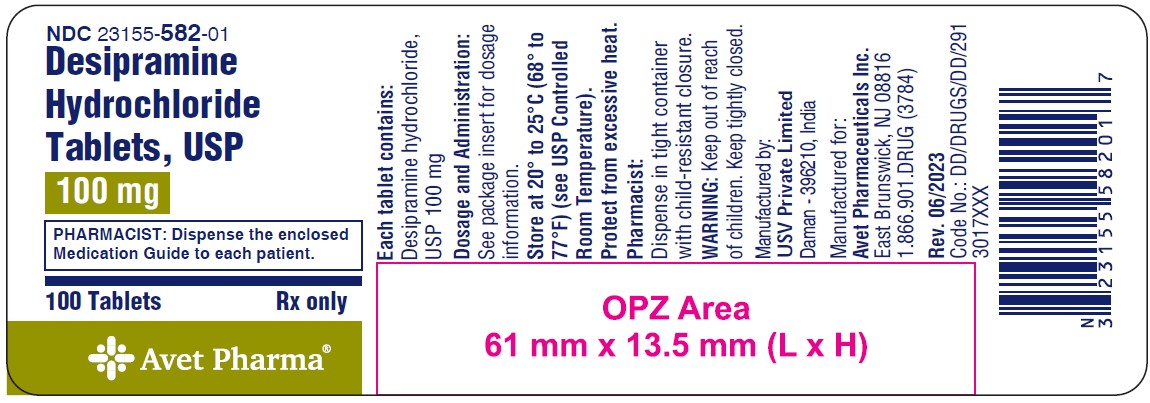
NDC 23155-582-01
Desipramine Hydrochloride Tablets, USP
100 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
100 Tablets
Rx only
Avet Pharma Ltd.
NDC 23155-583-25
Desipramine Hydrochloride Tablets, USP
150 mg
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
50 tablets
Rx only
Avet Pharma Ltd.

BOXED WARNING SECTION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of desipramine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Desipramine hydrochloride is not approved for use in pediatric patients (SeeWARNINGS: Clinical Worsening and Suicide Risk**, PRECAUTIONS: Information for Patients, andPRECAUTIONS: Pediatric Use.)**
HOW SUPPLIED SECTION
HOW SUPPLIED
Desipramine hydrochloride tablets, USP for oral administration are available as:
10 mg: white to off white, rounded square shape, biconvex, coated tablets with 'N6' debossed on one side and plain on other side.
NDC 23155-578-01: Bottles of 100
25 mg: white to off white, round biconvex, coated tablets with 'I86' debossed on one side and plain on other side.
NDC 23155-579-01: Bottles of 100
50 mg: white to off white, round biconvex, coated tablets with 'I82' debossed on one side and plain on other side.
NDC 23155-580-01: Bottles of 100
75 mg: white to off white, round biconvex, coated tablets with 'I83' debossed on one side and plain on other side.
NDC 23155-581-01: Bottles of 100
100 mg: white to off white, round biconvex, coated tablets with 'I84' debossed on one side and plain on other side.
NDC 23155-582-01: Bottles of 100
150 mg: white to off white, round biconvex, coated tablets with 'I85' debossed on one side and plain on other side.
NDC 23155-583-25: Bottles of 50
Store at 20°-25°C (68°-77°F); [see USP Controlled Room Temperature]. Protect from excessive heat. Dispense in tight container with child-resistant closure.
Dispense with Medication Guide available at:www.avetpharma.com/product
Manufactured by:
USV Private Limited
Daman - 396210, India
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 06/2023
Dispense with Medication Guide available at:www.avetpharma.com/product
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Desipramine Hydrochloride Tablets, USP
(des-IP-ra-meen HYE-droe-KLOR-ide)
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with your, or your family member's, antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1.Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment. 2.Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions. 3.How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member? * Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed. * Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings. * Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Who should not take desipramine hydrochloride tablets, USP?
- You should not take desipramine hydrochloride tablets, USP if you take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 2 weeks of stopping desipramine hydrochloride tablets, USP unless directed to do so by your physician.
- Do not start desipramine hydrochloride tablets, USP if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Visual Problems
- eye pain
- changes in vision
- swelling or redness in or around the eye
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
What else do I need to know about antidepressant medicines?
*Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms. *Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants. *Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member. *Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider. *Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information. *Tell your healthcare provider if you are pregnant or plan to become pregnant during treatment with desipramine hydrochloride tablets, USP. * If you become pregnant during treatment with desipramine hydrochloride tablets USP, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1800-FDA-1088.
You may also report side effects to Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784)
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Dispense with Medication Guide available at: www.avetpharma.com/product
Manufactured by:
USV Private Limited
Daman - 396210, India
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 06/2023
DESCRIPTION SECTION
DESCRIPTION
Desipramine hydrochloride, USP is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz [b,ƒ] azepine-5-propanamine,10,11-dihydro- N-methyl-, monohydrochloride.
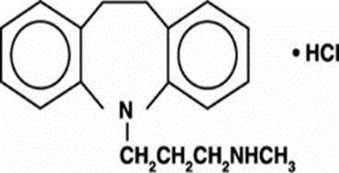
Each desipramine hydrochloride, USP tablet contains 10 mg, 25 mg, 50 mg, 75 mg, 100 mg, or 150 mg of desipramine hydrochloride for oral administration.
Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: Hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate type A (potato), stearic acid, talc and titanium dioxide.
