Losartan Potassium
These highlights do not include all the information needed to use LOSARTAN POTASSIUM TABLETS safely and effectively. See full prescribing information for LOSARTAN POTASSIUM TABLETS. LOSARTAN POTASSIUM Tablets, for oral use Initial U.S. Approval:1995
92691768-b5e6-4527-98dc-d86c58f6f13e
HUMAN PRESCRIPTION DRUG LABEL
Sep 2, 2025
Unichem Pharmaceuticals (USA), Inc.
DUNS: 181620514
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Losartan Potassium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Losartan Potassium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Losartan Potassium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL



ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypertension
Losartan potassium has been evaluated for safety in more than 3300 adult patients treated for essential hypertension and 4058 patients/subjects overall. Over 1200 patients were treated for over 6 months and more than 800 for over one year.
Treatment with losartan potassium was well-tolerated with an overall incidence of adverse events similar to that of placebo. In controlled clinical trials, discontinuation of therapy for adverse events occurred in 2.3% of patients treated with losartan potassium and 3.7% of patients given placebo. In 4 clinical trials involving over 1000 patients on various doses (10-150 mg) of losartan potassium and over 300 patients given placebo, the adverse events that occurred in ≥2% of patients treated with losartan potassium and more commonly than placebo were: dizziness (3% vs. 2%), upper respiratory infection (8% vs. 7%), nasal congestion (2% vs. 1%), and back pain (2% vs. 1%).
The following less common adverse reactions have been reported:
Blood and lymphatic system disorders: Anemia.
Psychiatric disorders: Depression.
Nervous system disorders: Somnolence, headache, sleep disorders, paresthesia, migraine.
Ear and labyrinth disorders: Vertigo, tinnitus.
Cardiac disorders: Palpitations, syncope, atrial fibrillation, CVA.
Respiratory, thoracic and mediastinal disorders: Dyspnea.
Gastrointestinal disorders: Abdominal pain, constipation, nausea, vomiting.
Skin and subcutaneous tissue disorders: Urticaria, pruritus, rash, photosensitivity.
Musculoskeletal and connective tissue disorders: Myalgia, arthralgia.
Reproductive system and breast disorders: Impotence.
General disorders and administration site conditions: Edema.
Cough
Persistent dry cough (with an incidence of a few percent) has been associated with ACE-inhibitor use and in practice can be a cause of discontinuation of ACE-inhibitor therapy. Two prospective, parallel-group, double-blind, randomized, controlled trials were conducted to assess the effects of losartan on the incidence of cough in hypertensive patients who had experienced cough while receiving ACE-inhibitor therapy. Patients who had typical ACE-inhibitor cough when challenged with lisinopril, whose cough disappeared on placebo, were randomized to losartan 50 mg, lisinopril 20 mg, or either placebo (one study, n=97) or 25 mg hydrochlorothiazide (n=135). The double-blind treatment period lasted up to 8 weeks. The incidence of cough is shown in Table 1 below.
Table 1
| |||
|
** Study 1******* |
** HCTZ** |
** Losartan** |
** Lisinopril** |
|
Cough |
25% |
17% |
69% |
|
** Study 2****†** |
** Placebo** |
** Losartan** |
** Lisinopril** |
|
Cough |
35% |
29% |
62% |
These studies demonstrate that the incidence of cough associated with losartan therapy, in a population that all had cough associated with ACE-inhibitor therapy, is similar to that associated with hydrochlorothiazide or placebo therapy.
Cases of cough, including positive re-challenges, have been reported with the use of losartan in postmarketing experience.
Hypertensive Patients with Left Ventricular Hypertrophy
In the Losartan Intervention for Endpoint (LIFE) study, adverse reactions with losartan potassium were similar to those reported previously for patients with hypertension.
Nephropathy in Type 2 Diabetic Patients
In the Reduction of Endpoints in NIDDM with the Angiotensin II Receptor Antagonist Losartan (RENAAL) study involving 1513 patients treated with losartan potassium or placebo, the overall incidences of reported adverse events were similar for the two groups. Discontinuations of losartan potassium because of side effects were similar to placebo (19% for losartan potassium, 24% for placebo). The adverse events, regardless of drug relationship, reported with an incidence of ≥ 4% of patients treated with losartan potassium and occurring with ≥ 2% difference in the losartan group vs. placebo on a background of conventional antihypertensive therapy, were asthenia/fatigue, chest pain, hypotension, orthostatic hypotension, diarrhea, anemia, hyperkalemia, hypoglycemia, back pain, muscular weakness, and urinary tract infection.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing experience with losartan potassium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure:
Digestive: Hepatitis.
General Disorders and Administration Site Conditions: Malaise.
Hematologic: Thrombocytopenia.
Hypersensitivity: Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, tongue, and/or swelling of the intestine has been reported; some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Vasculitis, including Henoch-Schönlein purpura, has been reported. Anaphylactic reactions have been reported.
Metabolic and Nutrition: Hyponatremia.
Musculoskeletal: Rhabdomyolysis.
Nervous System Disorders: Dysgeusia.
Skin: Erythroderma.
Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, and back pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Unichem at 1-866-562-4616 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
Losartan potassium USP is an angiotensin II receptor blocker acting on the AT1 receptor subtype. Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1Htetrazol-5ylphenyl)benzyl] imidazole-5-methanol monopotassium salt.
Its empirical formula is C22H22ClKN6O, and its structural formula is:
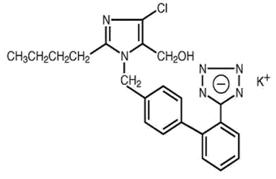
Losartan potassium USP is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan.
Losartan potassium is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium USP and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, pregelatinized starch, sodium starch glycolate, corn starch, magnesium stearate, hypromellose, polyethylene glycol, titanium dioxide, D&C yellow no. 10 aluminium lake and FD&C blue no. 2 aluminium lake.
Losartan potassium tablets, USP 25 mg, 50 mg and 100 mg contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hypertension
Adult Hypertension
The antihypertensive effects of losartan potassium tablets were demonstrated principally in 4 placebo-controlled, 6-to 12 week trials of dosages from 10 to 150 mg per day in patients with baseline diastolic blood pressures of 95-115. The studies allowed comparisons of two doses (50-100 mg/day) as once-daily or twice-daily regimens, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Three additional studies examined the antihypertensive effects of losartan and hydrochlorothiazide in combination.
The 4 studies of losartan monotherapy included a total of 1075 patients randomized to several doses of losartan and 334 to placebo. The 10-and 25-mg doses produced some effect at peak (6 hours after dosing) but small and inconsistent trough (24 hour) responses. Doses of 50, 100 and 150 mg once daily gave statistically significant systolic/diastolic mean decreases in blood pressure, compared to placebo in the range of 5.5-10.5 / 3.5-7.5 mmHg, with the 150-mg dose giving no greater effect than 50-100 mg. Twice-daily dosing at 50-100 mg/day gave consistently larger trough responses than once- daily dosing at the same total dose. Peak (6 hour) effects were uniformly, but moderately, larger than trough effects, with the trough-to-peak ratio for systolic and diastolic responses 50-95% and 60-90%, respectively.
Addition of a low dose of hydrochlorothiazide (12.5 mg) to losartan 50 mg once daily resulted in placebo-adjusted blood pressure reductions of 15.5/9.2 mmHg.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65, had generally similar responses. Losartan potassium tablets was effective in reducing blood pressure regardless of race, although the effect was somewhat less in Black patients (usually a low-renin population).
Pediatric Hypertension
The antihypertensive effect of losartan was studied in one trial enrolling 177 hypertensive pediatric patients aged 6 to 16 years old. Children who weighed <50 kg received 2.5, 25 or 50 mg of losartan daily and patients who weighed ≥50 kg received 5, 50 or 100 mg of losartan daily. Children in the lowest dose group were given losartan in a suspension formulation [see Dosage and Administration (2.1)]. The majority of the children had hypertension associated with renal and urogenital disease. The sitting diastolic blood pressure (SiDBP) on entry into the study was higher than the 95thpercentile level for the patient's age, gender, and height. At the end of three weeks, losartan reduced systolic and diastolic blood pressure, measured at trough, in a dose-dependent manner. Overall, the two higher doses (25 to 50 mg in patients <50 kg; 50 to 100 mg in patients ≥50 kg) reduced diastolic blood pressure by 5 to 6 mmHg more than the lowest dose used (2.5 mg in patients <50 kg; 5 mg in patients ≥50 kg). The lowest dose, corresponding to an average daily dose of 0.07 mg/kg, did not appear to offer consistent antihypertensive efficacy. When patients were randomized to continue losartan at the two higher doses or to placebo after 3 weeks of therapy, trough diastolic blood pressure rose in patients on placebo between 5 and 7 mmHg more than patients randomized to continuing losartan. When the low dose of losartan was randomly withdrawn, the rise in trough diastolic blood pressure was the same in patients receiving placebo and in those continuing losartan, again suggesting that the lowest dose did not have significant antihypertensive efficacy. Overall, no significant differences in the overall antihypertensive effect of losartan were detected when the patients were analyzed according to age (<, ≥12 years old) or gender. While blood pressure was reduced in all racial subgroups examined, too few non-White patients were enrolled to compare the dose- response of losartan in the non-White subgroup.
14.2 Hypertensive Patients with Left Ventricular Hypertrophy
The LIFE study was a multinational, double-blind study comparing losartan potassium and atenolol in 9193 hypertensive patients with ECG-documented left ventricular hypertrophy. Patients with myocardial infarction or stroke within six months prior to randomization were excluded. Patients were randomized to receive once daily losartan potassium tablets 50 mg or atenolol 50 mg. If goal blood pressure (<140/90 mmHg) was not reached, hydrochlorothiazide (12.5 mg) was added first and, if needed, the dose of losartan potassium or atenolol was then increased to 100 mg once daily. If necessary, other antihypertensive treatments (e.g., increase in dose of hydrochlorothiazide therapy to 25 mg or addition of other diuretic therapy, calcium-channel blockers, alpha-blockers, or centrally acting agents, but not ACE inhibitors, angiotensin II antagonists, or beta-blockers) were added to the treatment regimen to reach the goal blood pressure.
Of the randomized patients, 4963 (54%) were female and 533 (6%) were Black. The mean age was 67 with 5704 (62%) age ≥65. At baseline, 1195 (13%) had diabetes, 1326 (14%) had isolated systolic hypertension, 1469 (16%) had coronary heart disease, and 728 (8%) had cerebrovascular disease. Baseline mean blood pressure was 174/98 mmHg in both treatment groups. The mean length of follow-up was 4.8 years. At the end of study or at the last visit before a primary endpoint, 77% of the group treated with losartan potassium and 73% of the group treated with atenolol were still taking study medication. Of the patients still taking study medication, the mean doses of losartan potassium and atenolol were both about 80 mg/day, and 15% were taking atenolol or losartan as monotherapy, while 77% were also receiving hydrochlorothiazide (at a mean dose of 20 mg/day in each group). Blood pressure reduction measured at trough was similar for both treatment groups but blood pressure was not measured at any other time of the day. At the end of study or at the last visit before a primary endpoint, the mean blood pressures were 144.1/81.3 mmHg for the group treated with losartan potassium and 145.4/80.9 mmHg for the group treated with atenolol; the difference in systolic blood pressure (SBP) of 1.3 mmHg was significant (p<0.001), while the difference of 0.4 mmHg in diastolic blood pressure (DBP) was not significant (p=0.098).
The primary endpoint was the first occurrence of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. Patients with nonfatal events remained in the trial, so that there was also an examination of the first event of each type even if it was not the first event (e.g., a stroke following an initial myocardial infarction would be counted in the analysis of stroke). Treatment with losartan potassium resulted in a 13% reduction (p=0.021) in risk of the primary endpoint compared to the atenolol group (see Figure 1 and Table 3); this difference was primarily the result of an effect on fatal and nonfatal stroke. Treatment with losartan potassium reduced the risk of stroke by 25% relative to atenolol (p=0.001) (see Figure 2 and Table 3).
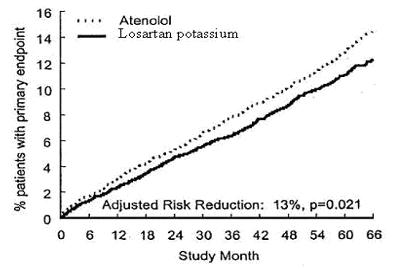
**Figure 1:**Kaplan-Meier estimates of the primary endpoint of time to cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy
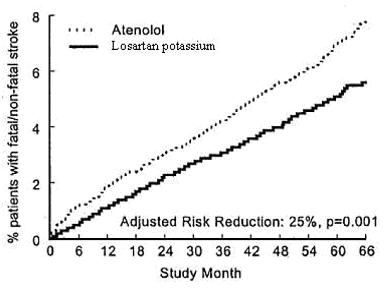
**Figure 2:**Kaplan-Meier estimates of the time to fatal/nonfatal stroke in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.
Table 3 shows the results for the primary composite endpoint and the individual endpoints. The primary endpoint was the first occurrence of stroke, myocardial infarction or cardiovascular death, analyzed using an ITT approach. The table shows the number of events for each component in two different ways. The Components of Primary Endpoint (as a first event) counts only the events that define the primary endpoint, while the Secondary Endpoints count all first events of a particular type, whether or not they were preceded by a different type of event.
Table 3: Incidence of Primary Endpoint Events
| |||||||
|
** Losartan potassium** |
** Atenolol** |
** Risk Reduction******* |
** 95% CI** |
** p-Value** | |||
|
** N (%)** |
** Rate****†** |
** N (%)** |
** Rate****†** | ||||
|
Primary Composite Endpoint |
508 (11) |
23.8 |
588 (13) |
27.9 |
13% |
2% to 23% |
0.021 |
|
Components of Primary Composite Endpoint (as a first event) | |||||||
|
Stroke (nonfatal) |
209 (5) |
286 (6) | |||||
|
Myocardial infarction (nonfatal) |
174 (4) |
168 (4) | |||||
|
Cardiovascular mortality |
125 (3) |
134 (3) | |||||
|
Secondary Endpoints (any time in study) | |||||||
|
Stroke (fatal/nonfatal) |
232 (5) |
10.8 |
309 (7) |
14.5 |
25% |
11% to 37% |
0.001 |
|
Myocardial infarction (fatal/nonfatal) |
198 (4) |
9.2 |
188 (4) |
8.7 |
-7% |
-13% to 12% |
0.491 |
|
Cardiovascular mortality |
204 (4) |
9.2 |
234 (5) |
10.6 |
11% |
-7% to 27% |
0.206 |
|
Due to CHD |
125 (3) |
5.6 |
124 (3) |
5.6 |
-3% |
-32% to 20% |
0.839 |
|
Due to Stroke |
40 (1) |
1.8 |
62 (1) |
2.8 |
35% |
4% to 67% |
0.032 |
|
Other‡ |
39 (1) |
1.8 |
48 (1) |
2.2 |
16% |
-28% to 45% |
0.411 |
Although the LIFE study favored losartan potassium over atenolol with respect to the primary endpoint (p=0.021), this result is from a single study and, therefore, is less compelling than the difference between losartan potassium and placebo. Although not measured directly, the difference between losartan potassium and placebo is compelling because there is evidence that atenolol is itself effective (vs. placebo) in reducing cardiovascular events, including stroke, in hypertensive patients.
Other clinical endpoints of the LIFE study were: total mortality, hospitalization for heart failure or angina pectoris, coronary or peripheral revascularization procedures, and resuscitated cardiac arrest. There were no significant differences in the rates of these endpoints between the losartan potassium and atenolol groups.
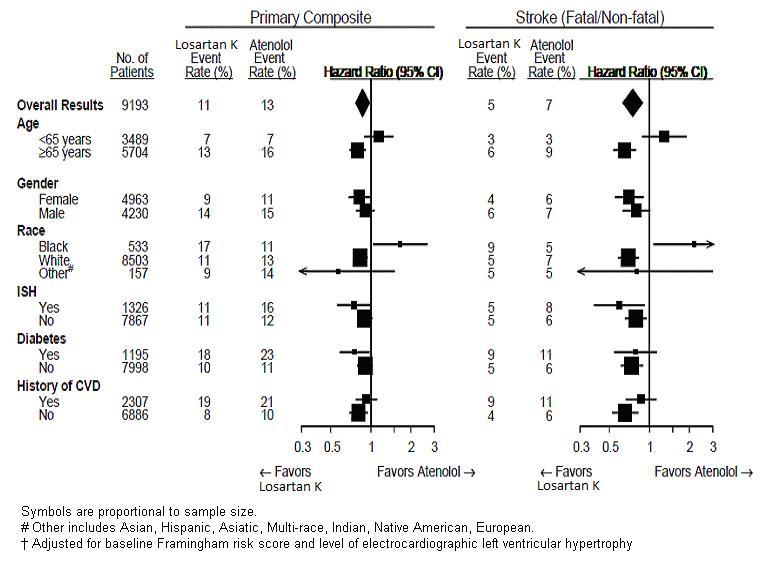
For the primary endpoint and stroke, the effects of losartan potassium in patient subgroups defined by age, gender, race and presence or absence of isolated systolic hypertension (ISH), diabetes, and history of cardiovascular disease (CVD) are shown in Figure 3 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
Figure 3: Primary Endpoint Events**†**within Demographic Subgroups
14.3 Nephropathy in Type 2 Diabetic Patients
The RENAAL study was a randomized, placebo-controlled, double-blind, multicenter study conducted worldwide in 1513 patients with type 2 diabetes with nephropathy (defined as serum creatinine 1.3 to 3.0 mg/dL in females or males ≤60 kg and 1.5 to 3.0 mg/dL in males >60 kg and proteinuria [urinary albumin to creatinine ratio ≥300 mg/g]).
Patients were randomized to receive losartan potassium tablets, 50 mg once daily or placebo on a background of conventional antihypertensive therapy excluding ACE inhibitors and angiotensin II antagonists. After one month, investigators were instructed to titrate study drug to 100 mg once daily if the trough blood pressure goal (140/90 mmHg) was not achieved. Overall, 72% of patients received the 100-mg daily dose more than 50% of the time they were on study drug. Because the study was designed to achieve equal blood pressure control in both groups, other antihypertensive agents (diuretics, calcium- channel blockers, alpha-or beta-blockers, and centrally acting agents) could be added as needed in both groups. Patients were followed for a mean duration of 3.4 years.
The study population was diverse with regard to race (Asian 16.7%, Black 15.2%, Hispanic 18.3%, White 48.6%). Overall, 63.2% of the patients were men, and 66.4% were under the age of 65 years. Almost all of the patients (96.6%) had a history of hypertension, and the patients entered the trial with a mean serum creatinine of 1.9 mg/dL and mean proteinuria (urinary albumin/creatinine) of 1808 mg/g at baseline.
The primary endpoint of the study was the time to first occurrence of any one of the following events: doubling of serum creatinine, end-stage renal disease (ESRD) (need for dialysis or transplantation), or death. Treatment with losartan potassium resulted in a 16% risk reduction in this endpoint (see Figure 4 and Table 4). Treatment with losartan potassium also reduced the occurrence of sustained doubling of serum creatinine by 25% and ESRD by 29% as separate endpoints, but had no effect on overall mortality (see Table 4).
The mean baseline blood pressures were 152/82 mmHg for losartan potassium plus conventional antihypertensive therapy and 153/82 mmHg for placebo plus conventional antihypertensive therapy. At the end of the study, the mean blood pressures were 143/76 mmHg for the group treated with losartan potassium and 146/77 mmHg for the group treated with placebo.
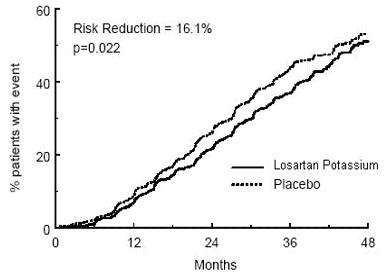
**Figure 4:**Kaplan-Meier curve for the primary composite endpoint of doubling of serum creatinine, end stage renal disease (need for dialysis or transplantation) or death.
Table 4: Incidence of Primary Endpoint Events|
** Incidence** |
** Risk Reduction** |
** 95% C.I.** |
** p-Value** | ||
|
** Losartan** |
** Placebo** | ||||
|
Primary Composite Endpoint |
43.5% |
47.1% |
16.1% |
2.3% to 27.9% |
0.022 |
|
Doubling of Serum Creatinine, ESRD and Death Occurring as a First Event | |||||
|
Doubling of Serum Creatinine |
21.6% |
26.0% | |||
|
ESRD |
8.5% |
8.5% | |||
|
Death |
13.4% |
12.6% | |||
|
Overall Incidence of Doubling of Serum Creatinine, ESRD and Death | |||||
|
Doubling of Serum Creatinine |
21.6% |
26.0% |
25.3% |
7.8% to 39.4% |
0.006 |
|
ESRD |
19.6% |
25.5% |
28.6% |
11.5% to 42.4% |
0.002 |
|
Death |
21.0% |
20.3% |
-1.7% |
-26.9% to 18.6% |
0.884 |
The secondary endpoints of the study were change in proteinuria, change in the rate of progression of renal disease, and the composite of morbidity and mortality from cardiovascular causes (hospitalization for heart failure, myocardial infarction, revascularization, stroke, hospitalization for unstable angina, or cardiovascular death). Compared with placebo, losartan potassium significantly reduced proteinuria by an average of 34%, an effect that was evident within 3 months of starting therapy, and significantly reduced the rate of decline in glomerular filtration rate during the study by 13%, as measured by the reciprocal of the serum creatinine concentration. There was no significant difference in the incidence of the composite endpoint of cardiovascular morbidity and mortality.
The favorable effects of losartan potassium were seen in patients also taking other anti-hypertensive medications (angiotensin II receptor antagonists and angiotensin converting enzyme inhibitors were not allowed), oral hypoglycemic agents and lipid-lowering agents.
For the primary endpoint and ESRD, the effects of losartan potassium in patient subgroups defined by age, gender and race are shown in Table 5 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
Table 5: Efficacy Outcomes within Demographic Subgroups|
** No. of Patients** |
** Primary Composite Endpoint** |
** ESRD** | |||||
|
** Losartan potassium Event Rate** |
** Placebo Event Rate %** |
** Hazard Ratio (95% CI)** |
** Losartan potassium Event Rate** |
** Placebo Event Rate %** |
** Hazard Ratio (95% CI)** | ||
|
Overall Results |
1513 |
43.5 |
47.1 |
0.84 (0.72, 0.98) |
19.6 |
25.5 |
0.71 (0.58, 0.89) |
|
Age | |||||||
|
<65 years |
1005 |
44.1 |
49.0 |
0.78 (0.65, 0.94) |
21.1 |
28.5 |
0.67 (0.52, 0.86) |
|
≥65 years |
508 |
42.3 |
43.5 |
0.98 (0.75, 1.28) |
16.5 |
19.6 |
0.85 (0.56, 1.28) |
|
Gender | |||||||
|
Female |
557 |
47.8 |
54.1 |
0.76 (0.60, 0.96) |
22.8 |
32.8 |
0.60 (0.44, 0.83) |
|
Male |
956 |
40.9 |
43.3 |
0.89 (0.73, 1.09) |
17.5 |
21.5 |
0.81 (0.60, 1.08) |
|
Race | |||||||
|
Asian |
252 |
41.9 |
54.8 |
0.66 (0.45, 0.95) |
18.8 |
27.4 |
0.63 (0.37, 1.07) |
|
Black |
230 |
40.0 |
39.0 |
0.98 (0.65, 1.50) |
17.6 |
21.0 |
0.83 (0.46, 1.52) |
|
Hispanic |
277 |
55.0 |
54.0 |
1.00 (0.73, 1.38) |
30.0 |
28.5 |
1.02 (0.66, 1.59) |
|
White |
735 |
40.5 |
43.2 |
0.81 (0.65, 1.01) |
16.2 |
23.9 |
0.60 (0.43, 0.83) |
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Additional patient information leaflets can be obtained by calling Unichem at 1-866-562-4616.
Pregnancy
Advise female patients of childbearing age about the consequences of exposure to losartan potassium tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Potassium Supplements
Advise patients receiving losartan potassium tablets not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider [see Drug Interactions (7.1)].
The trademarks depicted herein are owned by their respective companies.
Manufactured by:
UNICHEM LABORATORIES LTD.
Ind. Area, Meerut Road, Ghaziabad – 201 003, India.
Manufactured for:

East Brunswick, NJ 08816
SPL MEDGUIDE SECTION
PATIENT INFORMATION
Losartan Potassium Tablets
(loe sar' tan poe tas' ee um)
25 mg, 50 mg, 100 mg
Rx only
Read the Patient Information that comes with losartan potassium tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment.
What is the most important information I should know about****losartan potassium tablets?
*Losartan potassium tablets can cause harm or death to an unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you get pregnant while taking losartan potassium tablets tell your doctor right away.
What are losartan potassium tablets?
Losartan potassium tablets are prescription medicine called an angiotensin receptor blocker (ARB). It is used:
- alone or with other blood pressure medicines to lower high blood pressure (hypertension).
- to lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy. Losartan potassium tablets may not help Black patients with this problem.
- to slow the worsening of diabetic kidney disease (nephropathy) in patients with type 2 diabetes who have or had high blood pressure.
Losartan potassium tablets has not been studied in children less than 6 years old or in children with certain kidney problems.
**High Blood Pressure (hypertension).**Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Losartan potassium tablets can help your blood vessels relax so your blood pressure is lower.
**Left Ventricular Hypertrophy (LVH)**is an enlargement of the walls of the left chamber of the heart (the heart's main pumping chamber). LVH can happen from several things. High blood pressure is the most common cause of LVH.
**Type 2 Diabetes with Nephropathy.**Type 2 diabetes is a type of diabetes that happens mainly in adults. If you have diabetic nephropathy it means that your kidneys do not work properly because of damage from the diabetes.
Who should not take losartan potassium tablets?
*Do not take losartan potassium tablets if you are allergic to any of the ingredients in losartan potassium tablets. See the end of this leaflet for a complete list of ingredients in losartan potassium tablets.
- Do not take losartan potassium tablets if you have diabetes and are taking a medicine called aliskiren to reduce blood pressure.
What should I tell my doctor before taking losartan potassium tablets?
Tell your doctor about all of your medical conditions including if you:
are pregnant or planning to become pregnant. See*"What is the most important information I should know about losartan potassium tablets?"** *are breastfeeding. It is not known if losartan potassium passes into your breast milk. You should choose either to take losartan potassium tablets or breastfeed, but not both.
- are vomiting a lot or having a lot of diarrhea
- have liver problems
- have kidney problems
**Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.**Losartan potassium tablets and certain other medicines may interact with each other. Especially tell your doctor if you are taking:
- potassium supplements
- salt substitutes containing potassium
- other medicines that may increase serum potassium
- water pills (diuretics)
- lithium (a medicine used to treat a certain kind of depression)
- medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors
- other medicines to reduce blood pressure
How should I take losartan potassium tablets?
- Take losartan potassium tablets exactly as prescribed by your doctor. Your doctor may change your dose if needed.
- Losartan potassium tablets can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much losartan potassium tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
What are the possible side effects of losartan potassium tablets?
Losartan potassium tablets may cause the following side effects that may be serious:
*Injury or death of unborn babies. See "What is the most important information I should know about losartan potassium tablets?" *Allergic reaction. Symptoms of an allergic reaction are swelling of the face, lips, throat or tongue. Get emergency medical help right away and stop taking losartan potassium tablets. *Low blood pressure (hypotension). Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away. *For people who already have kidney problems, you may see a worsening in how well your kidneys work. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
- High blood levels of potassium
The most common side effects of losartan potassium tablets in people with high blood pressure are:
- "colds" (upper respiratory infection)
- dizziness
- stuffy nose
- back pain
The most common side effects of losartan potassium tablets in people with type 2 diabetes with diabetic kidney disease are:
- diarrhea
- tiredness
- low blood sugar
- chest pain
- high blood potassium
- low blood pressure
Tell your doctor if you get any side effect that bothers you or that won't go away.
This isnota complete list of side effects. For a complete list, ask your doctor or pharmacist.
How do I store losartan potassium tablets?
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Keep losartan potassium tablets in a tightly closed container that protects the medicine from light. *Keep losartan potassium tablets and all medicines out of the reach of children.
General information about losartan potassium tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use losartan potassium tablets for a condition for which it was not prescribed. Do not give losartan potassium tablets to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about losartan potassium tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about losartan potassium tablets that is written for health professionals.
What are the ingredients in losartan potassium tablets?
**Active ingredients:**losartan potassium
Inactive ingredients:
microcrystalline cellulose, lactose monohydrate, pregelatinized starch, sodium starch glycolate, corn starch, magnesium stearate, hypromellose, polyethylene glycol, titanium dioxide, D&C yellow no. 10 aluminium lake and FD&C blue no. 2 aluminium lake.
Additional patient information leaflets can be obtained by calling Unichem at 1-866-562-4616.
Manufactured by:
UNICHEM LABORATORIES LTD.
Ind. Area, Meerut Road, Ghaziabad – 201 003, India.
Manufactured for:

East Brunswick, NJ 08816
15-R-08/2025
13016441
