Travoprost
These highlights do not include all the information needed to use TRAVOPROST OPHTHALMIC SOLUTION (Ionic Buffered Solution) safely and effectively. See full prescribing information for TRAVOPROST OPHTHALMIC SOLUTION (Ionic Buffered Solution).
98fb77b8-ee41-e2fc-7bcd-bc350e19cc10
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
travoprost
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
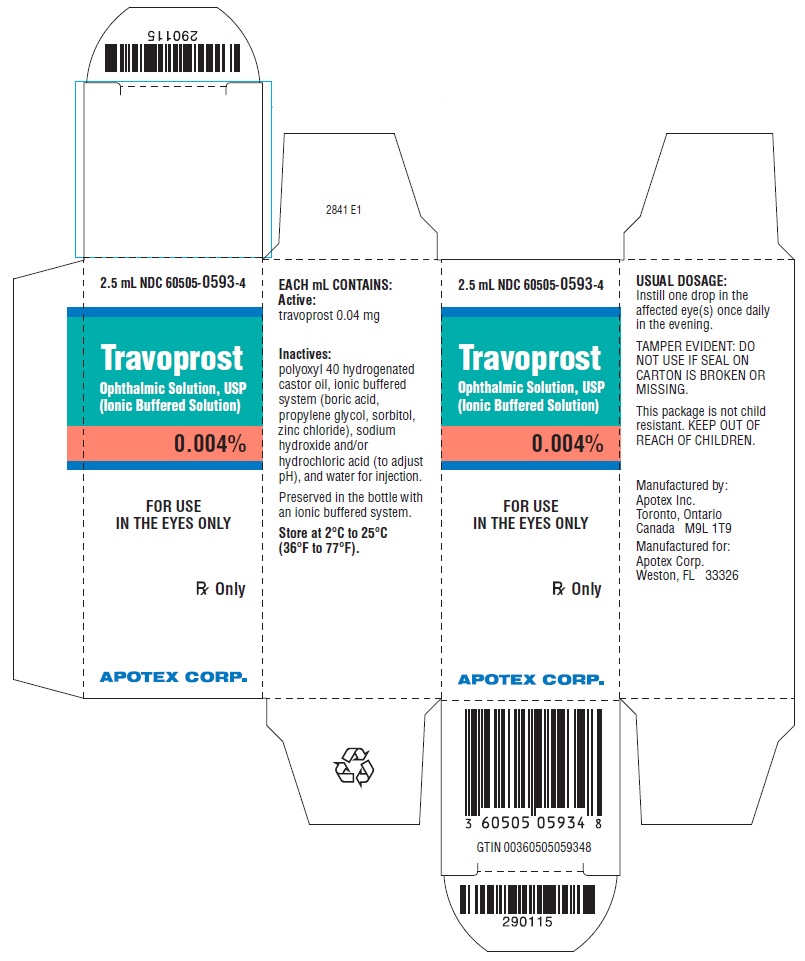
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CARTON LABEL - PRINCIPAL DISPLAY PANEL - 2.5 mL
APOTEX CORP. NDC 60505-0593-4
Travoprost Ophthalmic Solution, USP (Ionic Buffered Solution) 0.004%
Equivalent to 0.04 mg travoprost
Rx Only
DESCRIPTION SECTION
11 DESCRIPTION
Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1α(Z),2β(1E,3R*),3α,5α]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular formula of C26H35F3O6 and a molecular weight of 500.55 g/mol. The chemical structure of travoprost is:
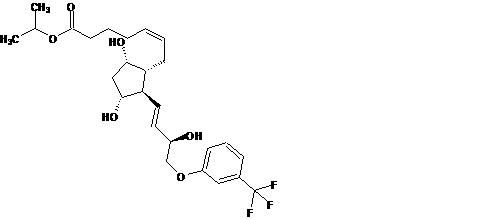
Travoprost is a clear, colorless to slightly yellow oil that is very soluble in acetonitrile, methanol, octanol, and chloroform. It is practically insoluble in water.
Travoprost ophthalmic solution, USP (ionic buffered solution) 0.004% is supplied as sterile, buffered aqueous solution of travoprost with a pH of approximately 5.7 and an osmolality of approximately 290 mOsmol/kg.
Travoprost ophthalmic solution, USP (ionic buffered solution) contains Active: travoprost 0.04 mg/mL;Inactives: polyoxyl 40 hydrogenated castor oil, ionic buffered system (boric acid, propylene glycol, sorbitol, zinc chloride), sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection. Preserved in the bottle with an ionic buffered system.
