Nystop
Nystatin Topical Powder, USP For topical use only.Not for ophthalmic use.
69ebb4dd-f45d-448d-97cd-3f8935493975
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nystatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
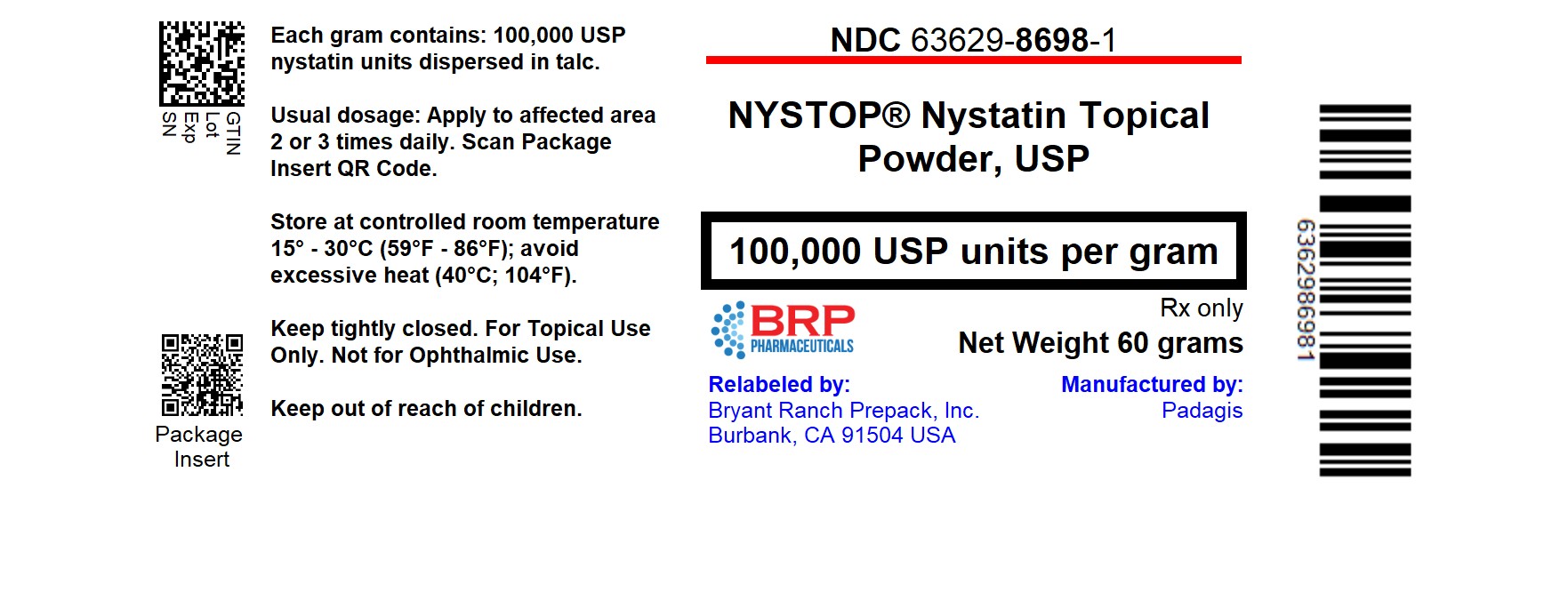
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nystatin 100000 unit/gram Powder, #60
DESCRIPTION SECTION
DESCRIPTION
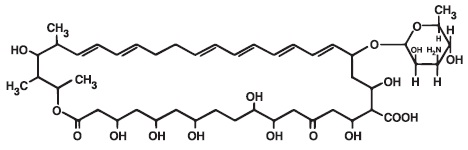
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei. The molecular formula for Nystatin is C47H75NO17. The molecular weight of Nystatin is 926.1.
Structural formula:
Nystatin Topical Powder USP is for dermatologic use.
Nystatin Topical Powder USP contains 100,000 USP nystatin units per gram dispersed in talc.
HOW SUPPLIED SECTION
HOW SUPPLIED
Nystop® Nystatin Topical Powder USP is supplied as 100,000 units nystatin per gram in 60 g plastic squeeze bottles.
(NDC 63629-8698-1)
STORAGE
Store at controlled room temperature 15°-30°C (59°-86°F); avoid excessive heat (40°C; 104°F).
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
