BREVIBLOC
These highlights do not include all the information needed to use BREVIBLOC injection safely and effectively. See full prescribing information for BREVIBLOC injection.BREVIBLOC (Esmolol Hydrochloride) injection, for intravenous useInitial U.S. Approval: 1986
220a07b8-5c68-41a3-8705-fd91f14c50b4
HUMAN PRESCRIPTION DRUG LABEL
Jun 15, 2023
Baxter Healthcare Corporation
DUNS: 005083209
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
esmolol hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
esmolol hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

Container Label
LOT EXP
NDC 10019-668-10
BREVIBLOC
PREMIXED
Double Strength Injection
Esmolol Hydrochloride in Sodium Chloride
2,000 mg/100 mL (20 mg/mL)
100 mL
Iso-Osmotic • No Preservative Added
Single Intravenous Use Only
****Each mL contains 20 mg Esmolol Hydrochloride
USP 4.1 mg Sodium Chloride USP in Water
for Injection USP Buffered with 2.8 mg Sodium
Acetate Trihydrate USP and 0.546 mg Glacial
Acetic Acid USP pH adjusted with sodium
hydroxide and/or hydrochloric acid pH 5.0
(4.5-5.5) Sterile Nonpyrogenic
USUAL DOSAGESee package insert
CAUTIONSCheck for le aks by squee zing
container firmly If le aks are found discard as
sterility may be impaired Use only if solu tion is
clear colorless to light yellow
Discard unused portion
DO NOT INTRODUCE ADDITIVES
****Must not be used in series connections
Store at 25°C (77°F) Excursions permitted to
15°-30°C (59°-86°F) [See USP Controlled Roo m Temperature ] PROTECT FROM FREEZING
Avoid excessive heat Rx only
MANUFACTURED BY****
****Baxter Logo
USA
novaplus logo
460-324-012J1416

Container Label
LOT EXP
NDC 10019-672-10
BREVIBLOC
PREMIXED
Double Strength Injection
Esmolol Hydrochloride in Sodium Chloride
2,500 mg/250 mL
(10 mg/mL)
250 mL
Iso-Osmotic
No Preservative Added
Single Intravenous Use Only
EACH mL CONTAINS 10 mg ESMOLOL
HYDROCHLORIDE USP 5.9 mg SODIUM
CHLORIDE USP IN WATER FOR INJECTION
USP BUFFERED WITH 2.8 mg SODIUM
ACETATE TRIHYDRATE USP AND 0.546 mg GLACIAL ACETIC ACID
USP PH ADJUSTED WITH SODIUM HYDROXIDE AND/OR HYDROCHLORIC ACID
PH 5.0 (4.5-5.5) STERILE NONPYROGENIC
USUAL DOSAGESEE PACKAGE INSERT
CAUTIONSCHECK FOR LE AKS BY SQUEEZING CONTAINER FIRMLY
IF LEAKS ARE FOUND DISCARD AS STERILITY MAY BE IMPAIRED USE
ONLY IF SOLUTION IS CLEAR COLORLESS TO LIGHT YELLOW DISCARD
UNUSED PORTION
DO NOT INTRODUCE ADDITIVES
****MUST NOT BE USED IN SERIES CONNECTIONS
STORE AT 25°C (77°F) EXCURSIONS PERMITTED TO 15°-30°C
(59°-86°F) [SEE USP CONTROLLED ROOM TEMPERATURE]
PROTECT FROM FREEZING AVOID EXCESSIVE HEAT RX ONLY
MANUFACTURED BY****
****BAXTER LOGO
****novaplus logo
USA
INTRAVIA CONTAINER
460-327-022J1417
****FOR PRODUCT INQUIRY
1 800 ANA DRUG
(1-800-262-3784)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosing for the Treatment of Supraventricular Tachycardia or
Noncompensatory Sinus Tachycardia
BREVIBLOC injection is administered by continuous intravenous infusion with or without a loading dose. Additional loading doses and/or titration of the maintenance infusion (step-wise dosing) may be necessary based on desired ventricular response.
Table 1 Step-Wise Dosing|
Step |
Action |
|
1 |
Optional loading dose (500 mcg per kg over 1 minute), then 50 mcg per kg per min for 4 min |
|
2 |
Optional loading dose if necessary, then 100 mcg per kg per min for 4 min |
|
3 |
Optional loading dose if necessary, then 150 mcg per kg per min for 4 min |
|
4 |
If necessary, increase dose to 200 mcg per kg per min |
In the absence of loading doses, continuous infusion of a single concentration of esmolol reaches pharmacokinetic and pharmacodynamic steady-state in about 30 minutes.
The effective maintenance dose for continuous and step-wise dosing is 50 to 200 mcg per kg per minute, although doses as low as 25 mcg per kg per minute have been adequate. Dosages greater than 200 mcg per kg per minute provide little added heart rate lowering effect, and the rate of adverse reactions increases.
Maintenance infusions may be continued for up to 48 hours.
2.2 Intraoperative and Postoperative Tachycardia and Hypertension
In this setting it is not always advisable to slowly titrate to a therapeutic effect. Therefore two dosing options are presented: immediate control and gradual control.
Immediate Control
•
Administer 1 mg per kg as a bolus dose over 30 seconds followed by an infusion of 150 mcg per kg per min if necessary.
•
Adjust the infusion rate as required to maintain desired heart rate and blood pressure. Refer to Maximum Recommended Doses below.
Gradual Control
•
Administer 500 mcg per kg as a bolus dose over 1 minute followed by a maintenance infusion of 50 mcg per kg per min for 4 minutes.
•
Depending on the response obtained, continue dosing as outlined for supraventricular tachycardia. Refer to Maximum Recommended Doses below.
Maximum Recommended Doses
•
For the treatment of tachycardia, maintenance infusion dosages greater than 200 mcg per kg per min are not recommended; dosages greater than 200 mcg per kg per min provide little additional heart rate-lowering effect, and the rate of adverse reactions increases.
•
For the treatment of hypertension, higher maintenance infusion dosages (250-300 mcg per kg per min) may be required. The safety of doses above 300 mcg per kg per minute has not been studied.
2.3 Transition from BREVIBLOC Injection Therapy to Alternative Drugs
After patients achieve adequate control of the heart rate and a stable clinical status, transition to alternative antiarrhythmic drugs may be accomplished.
When transitioning from BREVIBLOC injection to alternative drugs, the physician should carefully consider the labeling instructions of the alternative drug selected and reduce the dosage of BREVIBLOC injection as follows:
Thirty minutes following the first dose of the alternative drug, reduce the BREVIBLOC infusion rate by one-half (50%).
After administration of the second dose of the alternative drug, monitor the patient’s response and if satisfactory control is maintained for the first hour, discontinue the BREVIBLOC infusion.
2.4 Directions for Use
BREVIBLOC injection is available in a pre-mixed bag and ready-to-use vial. BREVIBLOC injection is not compatible with Sodium Bicarbonate (5%) solution (limited stability) or furosemide (precipitation).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Premixed Bag
•
The medication port is to be used solely for withdrawing an initial bolus from the bag.
•
Use aseptic technique when withdrawing the bolus dose.
•
Do not add any additional medications to the bag.
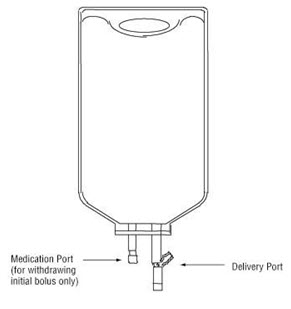
Figure 1: Two-Port INTRAVIA Bag
Ready-to-Use Vial
The Ready-to-use Vial may be used to administer a loading dosage by hand-held syringe while the maintenance infusion is being prepared [see How Supplied/Storage and Handling (16.2)].
Compatibility with Commonly Used Intravenous Fluids
BREVIBLOC injection was tested for compatibility with ten commonly used intravenous fluids at a final concentration of 10 mg esmolol hydrochloride per mL. BREVIBLOC injection was found to be compatible with the following solutions and was stable for at least 24 hours at controlled room temperature or under refrigeration:
•
Dextrose (5%) Injection, USP
•
Dextrose (5%) in Lactated Ringer’s Injection
•
Dextrose (5%) in Ringer’s Injection
•
Dextrose (5%) and Sodium Chloride (0.45%) Injection, USP
•
Dextrose (5%) and Sodium Chloride (0.9%) Injection, USP
•
Lactated Ringer’s Injection, USP
•
Potassium Chloride (40 mEq/liter) in Dextrose (5%) Injection, USP
•
Sodium Chloride (0.45%) Injection, USP
•
Sodium Chloride (0.9%) Injection, USP
•
Administer intravenously (2.1, 2.2)
•
Titrate using ventricular rate or blood pressure at ≥4-minute intervals. (2.1, 2.2)
•
Supraventricular tachycardia (SVT) or noncompensatory sinus tachycardia (2.1)
•
Optional loading dose: 500 mcg per kg infused over one minute
•
Then 50 mcg per kg per minute for the next 4 minutes
•
Adjust dose as needed to a maximum of 200 mcg per kg per minute.
•
Additional loading doses may be administered
•
Perioperative tachycardia and hypertension (2.2)
•
Loading dose: 500 mcg per kg over 1 minute for gradual control (1 mg per kg over 30 seconds for immediate control)
•
Then 50 mcg per kg per min for gradual control (150 mcg per kg per minute for immediate control) adjusted to a maximum of 200 (tachycardia) or 300 (hypertension) mcg per kg per min (2.2)
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
Overdoses of BREVIBLOC (Esmolol Hydrochloride) injection can cause cardiac and central nervous system effects. These effects may precipitate severe signs, symptoms, sequelae, and complications (for example, severe cardiac and respiratory failure, including shock and coma), and may be fatal. Continuous monitoring of the patient is required.
•
Cardiac effects include bradycardia, atrioventricular block (1st -, 2nd -, 3rd degree), junctional rhythms, intraventricular conduction delays, decreased cardiac contractility, hypotension, cardiac failure (including cardiogenic shock), cardiac arrest/asystole, and pulseless electrical activity.
•
Central nervous system effects include respiratory depression, seizures, sleep and mood disturbances, fatigue, lethargy, and coma.
•
In addition, bronchospasm, mesenteric ischemia, peripheral cyanosis, hyperkalemia, and hypoglycemia (especially in children) may occur.
10.2 Treatment Recommendations
Because of its approximately 9-minute elimination half-life, the first step in the management of toxicity should be to discontinue the BREVIBLOC infusion. Then, based on the observed clinical effects, consider the following general measures.
Bradycardia
Consider intravenous administration of atropine or another anticholinergic drug or cardiac pacing.
Cardiac Failure
Consider intravenous administration of a diuretic or digitalis glycoside. In shock resulting from inadequate cardiac contractility, consider intravenous administration of dopamine, dobutamine, isoproterenol, or inamrinone. Glucagon has been reported to be useful.
Symptomatic hypotension
Consider intravenous administration of fluids or vasopressor agents such as dopamine or norepinephrine.
Bronchospasm
Consider intravenous administration of a beta2 stimulating agent or a theophylline derivative.
10.3 Dilution Errors
Massive accidental overdoses of BREVIBLOC injection have resulted from dilution errors. Use of BREVIBLOC PREMIXED Injection and BREVIBLOC PREMIXED Double Strength Injection may reduce the potential for dilution errors. Some of these overdoses have been fatal while others resulted in permanent disability. Bolus doses in the range of 625 mg to 2.5 g (12.5‑50 mg/kg) have been fatal. Patients have recovered completely from overdoses as high as 1.75 g given over one minute or doses of 7.5 g given over one hour for cardiovascular surgery. The patients who survived appear to be those whose circulation could be supported until the effects of BREVIBLOC injection resolved.
DESCRIPTION SECTION
11 DESCRIPTION
BREVIBLOC (Esmolol Hydrochloride) injection is a beta adrenergic receptor blocker with a very short duration of action (elimination half-life is approximately 9 minutes). Esmolol hydrochloride is:
•
(±)-Methyl p-[2-hydroxy-3-(isopropylamino) propoxy] hydrocinnamate hydrochloride and has the following structure:
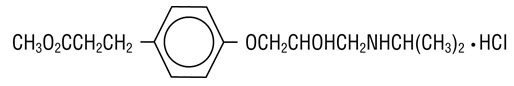
•
Esmolol hydrochloride has the empirical formula C16H26NO4Cl and a molecular weight of 331.8. It has one asymmetric center and exists as an enantiomeric pair.
•
Esmolol hydrochloride is a white to off-white crystalline powder. It is a relatively hydrophilic compound which is very soluble in water and freely soluble in alcohol. Its partition coefficient (octanol/water) at pH 7.0 is 0.42 compared to 17.0 for propranolol.
11.1 BREVIBLOC Injection Dosage Forms
All BREVIBLOC injection presentations are clear, colorless to light yellow, sterile, nonpyrogenic, iso‑osmotic solutions of esmolol hydrochloride in sodium chloride. The formulations for BREVIBLOC PREMIXED Injection, BREVIBLOC PREMIXED Double Strength Injection, and BREVIBLOC Injection are described in the table below:
Table 4 BREVIBLOC Injection Formulations|
BREVIBLOC PREMIXED Injection (Esmolol Hydrochloride) |
BREVIBLOC (Esmolol Hydrochloride) |
BREVIBLOC Injection (Esmolol Hydrochloride) | |
|
Esmolol Hydrochloride, USP |
10 mg/mL |
20 mg/mL |
10 mg/mL |
|
Sodium Chloride, USP |
5.9 mg/mL |
4.1 mg/mL |
5.9 mg/mL |
|
Water for |
Q.S. to volume |
Q.S. to volume |
Q.S. to volume |
|
Sodium Acetate Trihydrate, USP |
2.8 mg/mL |
2.8 mg/mL |
2.8 mg/mL |
|
Glacial Acetic |
0.546 mg/mL |
0.546 mg/mL |
0.546 mg/mL |
|
Sodium Hydroxide |
Q.S. to adjust pH to 4.5-5.5 | ||
|
Hydrochloric Acid |
Q.S. to adjust pH to 4.5-5.5 | ||
|
Q.S. = Quantity sufficient |
The calculated osmolarity of BREVIBLOC PREMIXED Injection and BREVIBLOC PREMIXED Double Strength Injection is 312 mOsmol/L. The 250 mL and 100 mL bags are non-latex, non-PVC INTRAVIA bags with dual PVC ports. The INTRAVIA bags are manufactured from a specially designed multilayer plastic (PL 2408). Solutions in contact with the plastic container leach out certain chemical compounds from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
BREVIBLOC PREMIXED Injection
•
NDC 10019-672-10, 2500 mg / 250 mL (10 mg/mL) Ready-to-use INTRAVIA Bags
BREVIBLOC PREMIXED Double Strength Injection
•
NDC 10019-668-10, 2000 mg / 100 mL (20 mg/mL) Ready-to-use INTRAVIA Bags
BREVIBLOC Injection
•
NDC 10019-115-01, 100 mg / 10 mL (10 mg/mL) Ready-to-use Vials, Package of 25
16.2 Storage
Store at 25˚C (77˚F). Excursions permitted to 15˚-30˚C (59˚-86˚F) [see USP Controlled Room Temperature]. Protect from freezing. Avoid excessive heat.
Each bag contains no preservative. Once drug has been withdrawn from ready-to- use bag, the bag should be used within 24 hours, with any unused portion discarded.
Visually inspect the container. If the administration port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired.
Do not use plastic containers in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
Do not remove unit from overwrap until ready to use. Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture barrier. The inner bag maintains sterility of the solution. Tear overwrap at notch and remove premixed bag. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard solution, as sterility may be impaired. Do not use unless the solution is clear (colorless to light yellow) and the seal is intact.
Preparation for intravenous administration:
•
Use aseptic technique.
•
Suspend premixed bag from eyelet support.
•
Remove plastic protector from delivery port at bottom of bag.
•
Attach administration set.
•
Refer to complete directions accompanying set.
