LEXIVA
These highlights do not include all the information needed to use LEXIVA safely and effectively. See full prescribing information for LEXIVA.LEXIVA (fosamprenavir calcium) tablets, for oral useLEXIVA (fosamprenavir calcium) oral suspensionInitial U.S. Approval: 2003
24feb9be-32a6-45fd-a896-f3e202edd8a9
HUMAN PRESCRIPTION DRUG LABEL
Oct 1, 2020
ViiV Healthcare Company
DUNS: 027295585
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fosamprenavir calcium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
fosamprenavir calcium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of LEXIVA/ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A, or initiation of medications metabolized by CYP3A in patients already receiving LEXIVA/ritonavir may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of LEXIVA/ritonavir, respectively. These interactions may lead to:
•
clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
•
clinically significant adverse reactions from greater exposures of LEXIVA/ritonavir.
•
loss of therapeutic effect of LEXIVA/ritonavir and possible development of resistance.
See Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during therapy with LEXIVA/ritonavir; review concomitant medications during therapy with LEXIVA/ritonavir, and monitor for the adverse reactions associated with the concomitant medications [see Contraindications (4), Drug Interactions (7)].
5.2 Skin Reactions
Severe and life-threatening skin reactions, including 1 case of Stevens- Johnson syndrome among 700 subjects treated with LEXIVA in clinical trials. Treatment with LEXIVA should be discontinued for severe or life-threatening rashes and for moderate rashes accompanied by systemic symptoms [see Adverse Reactions (6)].
5.3 Sulfa Allergy
LEXIVA should be used with caution in patients with a known sulfonamide allergy. Fosamprenavir contains a sulfonamide moiety. The potential for cross- sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown. In a clinical trial of LEXIVA used as the sole protease inhibitor, rash occurred in 2 of 10 subjects (20%) with a history of sulfonamide allergy compared with 42 of 126 subjects (33%) with no history of sulfonamide allergy. In 2 clinical trials of LEXIVA plus low-dose ritonavir, rash occurred in 8 of 50 subjects (16%) with a history of sulfonamide allergy compared with 50 of 412 subjects (12%) with no history of sulfonamide allergy.
5.4 Hepatic Toxicity
Use of LEXIVA with ritonavir at higher-than-recommended dosages may result in transaminase elevations and should not be used [see Dosage and Administration (2), Overdosage (10)]. Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for developing or worsening of transaminase elevations. Appropriate laboratory testing should be conducted prior to initiating therapy with LEXIVA and patients should be monitored closely during treatment.
5.5 Diabetes/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-1–infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between protease inhibitor therapy and these events have not been established.
5.6 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including LEXIVA. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain- Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.7 Increase in Body Fat
Increase of body fat has been observed in patients receiving protease inhibitors, including LEXIVA. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.8 Lipid Elevations
Treatment with LEXIVA plus ritonavir has resulted in increases in the concentration of triglycerides and cholesterol [see Adverse Reactions (6)]. Triglyceride and cholesterol testing should be performed prior to initiating therapy with LEXIVA and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate [see Drug Interactions (7)].
5.9 Hemolytic Anemia
Acute hemolytic anemia has been reported in a patient treated with amprenavir.
5.10 Patients with Hemophilia
There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients, additional factor VIII was required. In many of the reported cases, treatment with protease inhibitors was continued or restarted. A causal relationship between protease inhibitor therapy and these episodes has not been established.
5.11 Nephrolithiasis
Cases of nephrolithiasis were reported during postmarketing surveillance in HIV–1–infected patients receiving LEXIVA. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made. If signs or symptoms of nephrolithiasis occur, temporary interruption or discontinuation of therapy may be considered.
5.12 Resistance/Cross-Resistance
Because the potential for HIV cross-resistance among protease inhibitors has not been fully explored, it is unknown what effect therapy with LEXIVA will have on the activity of subsequently administered protease inhibitors. LEXIVA has been studied in patients who have experienced treatment failure with protease inhibitors [see Clinical Studies (14.2)].
•
The concomitant use of LEXIVA with ritonavir and certain other drugs may result in known or potentially significant drug interactions. Consult the full prescribing information prior to and during treatment for potential drug interactions. (5.1, 7.3)
•
LEXIVA should be discontinued for severe skin reactions including Stevens-Johnson syndrome. (5.2)
•
LEXIVA should be used with caution in patients with a known sulfonamide allergy. (5.3)
•
Use of higher-than-approved doses may lead to transaminase elevations. Patients with hepatitis B or C are at increased risk of transaminase elevations. (5.4)
•
Patients receiving LEXIVA may develop new onset or exacerbations of diabetes mellitus, hyperglycemia (5.5), immune reconstitution syndrome (5.6), increase of body fat (5.7), and elevated triglyceride and cholesterol concentrations (5.8). Monitor cholesterol and triglycerides prior to therapy and periodically thereafter.
•
Acute hemolytic anemia has been reported with amprenavir. (5.9)
•
Hemophilia: Spontaneous bleeding may occur, and additional factor VIII may be required. (5.10)
•
Nephrolithiasis: Cases of nephrolithiasis have been reported with fosamprenavir. (5.11)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Cytochrome P450 Inhibitors and Inducers
Amprenavir, the active metabolite of fosamprenavir, is an inhibitor of CYP3A4 metabolism and therefore should not be administered concurrently with medications with narrow therapeutic windows that are substrates of CYP3A4. Data also suggest that amprenavir induces CYP3A4.
Amprenavir is metabolized by CYP3A4. Coadministration of LEXIVA and drugs that induce CYP3A4, such as rifampin, may decrease amprenavir concentrations and reduce its therapeutic effect. Coadministration of LEXIVA and drugs that inhibit CYP3A4 may increase amprenavir concentrations and increase the incidence of adverse effects.
The potential for drug interactions with LEXIVA changes when LEXIVA is coadministered with the potent CYP3A4 inhibitor ritonavir. The magnitude of CYP3A4-mediated drug interactions (effect on amprenavir or effect on coadministered drug) may change when LEXIVA is coadministered with ritonavir. Because ritonavir is a CYP2D6 inhibitor, clinically significant interactions with drugs metabolized by CYP2D6 are possible when coadministered with LEXIVA plus ritonavir. Ritonavir also appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6, as well as other enzymes, including glucuronosyl transferase.
There are other agents that may result in serious and/or life-threatening drug interactions [see Contraindications (4)].
7.2 Established and Other Potentially Significant Drug Interactions
If LEXIVA is used in combination with ritonavir, see full prescribing information for ritonavir for additional information on drug interactions [see Contraindications (4), Clinical Pharmacology (12.3)].
Table 6 provides a listing of established or potentially clinically significant drug interactions. Information in the table applies to LEXIVA with or without ritonavir, unless otherwise indicated.
Table 6. Established and Other Potentially Significant Drug Interactions|
a See Clinical Pharmacology (12.3) Tables 10, 11, 12, or 13 for magnitude of interaction. | ||
|
Concomitant Drug Class: Drug Name |
Effect on Concentration of Amprenavir or Concomitant Drug |
Clinical Comment |
|
HCV/HIV-Antiviral Agents | ||
|
HCV protease inhibitor: Boceprevir |
LEXIVA: ↓Amprenavir (predicted) ↔ or ↓Boceprevir (predicted) LEXIVA/ritonavir: ↓Amprenavir (predicted) ↓Boceprevir (predicted) |
Coadministration of LEXIVA or LEXIVA/ritonavir and boceprevir is not recommended. |
|
HCV protease inhibitor: Simeprevir |
LEXIVA: ↔Amprenavir (predicted) ↑ or ↓Simeprevir (predicted) LEXIVA/ritonavir: ↔Amprenavir (predicted) ↑Simeprevir (predicted) |
Coadministration of LEXIVA or LEXIVA/ritonavir and simeprevir is not recommended. |
|
HCV protease inhibitor: Paritaprevir (coformulated with ritonavir and ombitasvir and coadministered with dasabuvir) |
LEXIVA: ↑Amprenavir (predicted) ↑ or ↔Paritaprevir (predicted) LEXIVA/ritonavir: ↑ or ↔Amprenavir (predicted) ↑Paritaprevir (predicted) |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. LEXIVA 1,400 mg once daily may be considered when coadministered with paritaprevir/ritonavir/ombitasvir/ dasabuvir. Coadministration of LEXIVA/ritonavir and paritaprevir/ritonavir/ombitasvir/ dasabuvir is not recommended. |
|
Non-nucleoside reverse transcriptase inhibitor: Delavirdinea |
LEXIVA: ↑Amprenavir ↓Delavirdine LEXIVA/ritonavir: ↑Amprenavir ↓Delavirdine |
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to delavirdine [see Contraindications (4)]. |
|
Non‑nucleoside reverse transcriptase inhibitor: Efavirenza |
LEXIVA: ↓Amprenavir LEXIVA/ritonavir: ↓Amprenavir |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. An additional 100 mg/day (300 mg total) of ritonavir is recommended when efavirenz is administered with LEXIVA/ritonavir once daily. No change in the ritonavir dose is required when efavirenz is administered with LEXIVA plus ritonavir twice daily. |
|
Non‑nucleoside reverse transcriptase inhibitor: Nevirapinea |
LEXIVA: ↓Amprenavir ↑Nevirapine LEXIVA/ritonavir: ↓Amprenavir ↑Nevirapine |
Coadministration of nevirapine and LEXIVA without ritonavir is not recommended. No dosage adjustment required when nevirapine is administered with LEXIVA/ritonavir twice daily. The combination of nevirapine administered with LEXIVA/ritonavir once‑daily regimen has not been studied. |
|
HIV protease inhibitor: Atazanavira |
LEXIVA: Interaction has not been evaluated. LEXIVA/ritonavir: ↓Atazanavir ↔Amprenavir |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitors: Indinavira, nelfinavira |
LEXIVA: ↑Amprenavir Effect on indinavir and nelfinavir is not well established. LEXIVA/ritonavir: Interaction has not been evaluated. |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitors: Lopinavir/ritonavira |
↓Amprenavir ↓Lopinavir |
An increased rate of adverse events has been observed. Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitor: Saquinavira |
LEXIVA: ↓Amprenavir Effect on saquinavir is not well established. LEXIVA/ritonavir: Interaction has not been evaluated. |
Appropriate doses of the combination with respect to safety and efficacy have not been established. |
|
HIV integrase inhibitor: Raltegravira |
LEXIVA: ↓Amprenavir ↓Raltegravir LEXIVA/ritonavir: ↓Amprenavir ↓Raltegravir |
Appropriate doses of the combination with respect to safety and efficacy have not been established. |
|
HIV integrase inhibitor: Dolutegravira |
LEXIVA/ritonavir: ↓Dolutegravir |
The recommended dose of dolutegravir is 50 mg twice daily when coadministered with LEXIVA/ritonavir. Use an alternative combination where possible in patients with known or suspected integrase inhibitor resistance. |
|
HIV CCR5 co-receptor antagonist: Maraviroca |
LEXIVA/ritonavir: ↓Amprenavir ↑Maraviroc |
No dosage adjustment required for LEXIVA/ritonavir. The recommended dose of maraviroc is 150 mg twice daily when coadministered with LEXIVA/ritonavir. LEXIVA should be given with ritonavir when coadministered with maraviroc. |
|
Other Agents | ||
|
Alpha 1-adrenoreceptor antagonist: Alfuzosin |
↑Alfuzosin |
Coadministration is contraindicated due to potential hypotension [see Contraindications (4)]. |
|
Antacid: MAALOX TC |
↓Amprenavir |
Use with caution when administered at the same time. LEXIVA may be less effective due to decreased amprenavir plasma concentrations. Staggered coadministration of antacids and LEXIVA has not been evaluated. |
|
Antiarrhythmics: Amiodarone, disopyramide, lidocaine (systemic), and quinidine |
↑Antiarrhythmics |
Therapeutic concentration monitoring, if available, is recommended for antiarrhythmics. |
|
Antiarrhythmics: Flecainide, propafenone |
↑Antiarrhythmics |
Coadministration is contraindicated due to potential for serious and/or life‑threatening reactions such as cardiac arrhythmias if LEXIVA is co- prescribed withritonavir[see Contraindications (4)]. |
|
Anticoagulant: Warfarin |
Concentrations of warfarin may be affected. It is recommended that INR (international normalized ratio) be monitored. | |
|
Anticonvulsants: Carbamazepine, phenobarbital, phenytoin |
LEXIVA: ↓Amprenavir |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations in patients taking these agents concomitantly. |
|
Phenytoina |
LEXIVA/ritonavir: ↑Amprenavir ↓Phenytoin |
Plasma phenytoin concentrations should be monitored and phenytoin dose should be increased as appropriate. No change in LEXIVA/ritonavir dose is recommended. |
|
Antidepressant: Paroxetine, trazodone |
↓Paroxetine |
Any paroxetine dose adjustment should be guided by clinical effect (tolerability and efficacy). |
|
↑Trazodone |
Adverse events of nausea, dizziness, hypotension, and syncope have been observed following coadministration of trazodone and ritonavir. If trazodone is used with a CYP3A4 inhibitor such as LEXIVA, the combination should be used with caution and a lower dose of trazodone should be considered. | |
|
Antifungals: Ketoconazolea, itraconazole |
↑Ketoconazole ↑Itraconazole |
Increase monitoring for adverse events. LEXIVA: Dose reduction of ketoconazole or itraconazole may be needed for patients receiving more than 400 mg ketoconazole or itraconazole per day. LEXIVA/ritonavir: High doses of ketoconazole or itraconazole (greater than 200 mg/day) are not recommended. |
|
Anti-gout: Colchicine |
↑Colchicine |
Patients with renal or hepatic impairment should not be given colchicine with LEXIVA/ritonavir. LEXIVA/ritonavir and coadministration of colchicine: Treatment of gout flares: 0.6 mg (1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Dose to be repeated no earlier than 3 days. Prophylaxis of gout flares: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted
to 0.3 mg once a day. Treatment of familial Mediterranean fever (FMF): Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day). LEXIVA and coadministration of colchicine: Treatment of gout flares: 1.2 mg (2 tablets) x 1 dose. Dose to be repeated no earlier than 3 days. Prophylaxis of gout flares: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted
to 0.3 mg twice a day or 0.6 mg once a day. Treatment of FMF: Maximum daily dose of 1.2 mg (may be given as 0.6 mg twice a day). |
|
Antimycobacterial: Rifabutina |
↑Rifabutin and rifabutin metabolite |
A complete blood count should be performed weekly and as clinically indicated to monitor for neutropenia. LEXIVA: A dosage reduction of rifabutin by at least half the recommended dose is required. LEXIVA/ritonavir: Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (a maximum dose of 150 mg every other day or 3 times per week). |
|
Antimycobacterial: Rifampina |
↓Amprenavir |
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to LEXIVA or to the class of protease inhibitors [see Contraindications (4)]. |
|
Antineoplastics: Dasatinib, nilotinib, ibrutinib, vinblastine, everolimus |
↑Antineoplastics |
LEXIVA or LEXIVA/ritonavir may increase plasma concentrations of antineoplastics metabolized by CYP3A, potentially increasing the risk of adverse events typically associated with these medications. In case of coadministration, please refer to relevant prescribing information for these medications. |
|
Antipsychotics: | ||
|
Lurasidone |
↑Lurasidone |
LEXIVA: If coadministration is necessary, reduce the lurasidone dose. Refer to the lurasidone prescribing information for concomitant use with moderate CYP3A4 inhibitors. LEXIVA/ritonavir: Use of lurasidone is contraindicated due to potential for serious and/or life- threatening reactions [see Contraindications (4)]. |
|
Pimozide |
↑Pimozide |
Coadministration is contraindicated due to potential for serious and/or life- threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
|
Quetiapine |
↑Quetiapine |
LEXIVA/ritonavir: Initiation of LEXIVA with ritonavir in patients taking quetiapine: Consider alternative antiretroviral therapy to avoid increases in quetiapine drug exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking LEXIVA with ritonavir: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. |
|
Benzodiazepines: Alprazolam, clorazepate, diazepam, flurazepam |
↑Benzodiazepines |
Clinical significance is unknown. A decrease in benzodiazepine dose may be needed. |
|
Calcium channel blockers: Diltiazem, felodipine, nifedipine, nicardipine, nimodipine, verapamil, amlodipine, nisoldipine, isradipine |
↑Calcium channel blockers |
Use with caution. Clinical monitoring of patients is recommended. |
|
Corticosteroid: Dexamethasone |
↓Amprenavir |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations. |
|
Endothelin‑receptor antagonist: Bosentan |
↑Bosentan |
Coadministration of bosentan in patients on LEXIVA: In patients who have been receiving LEXIVA for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability. Coadministration of LEXIVA in patients on bosentan: Discontinue use of bosentan at least 36 hours prior to initiation of LEXIVA. After at least 10 days following the initiation of LEXIVA, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability. |
|
Ergot derivatives: Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
↑Ergot derivatives |
Coadministration is contraindicated due to potential for serious and/or life‑threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [see Contraindications (4)]. |
|
GI motility agent: Cisapride |
↑Cisapride |
Coadministration is contraindicated due to potential for serious and/or life‑threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
|
Herbal product: St. John’s wort (Hypericum perforatum) |
↓Amprenavir |
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to LEXIVA or to the class of protease inhibitors [see Contraindications (4)]. |
|
Histamine H2-receptor antagonists: Cimetidine, famotidine, nizatidine, ranitidinea |
LEXIVA: ↓Amprenavir LEXIVA/ritonavir: Interaction not evaluated |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations. |
|
Immunosuppressants: Cyclosporine, tacrolimus, sirolimus |
↑Immunosuppressants |
Therapeutic concentration monitoring is recommended for immunosuppressant agents. |
|
Inhaled beta‑agonist: Salmeterol |
↑Salmeterol |
Concurrent administration of salmeterol with LEXIVA is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
|
Inhaled/nasal steroid: Fluticasone |
LEXIVA: ↑Fluticasone LEXIVA/ritonavir: ↑Fluticasone |
Use with caution. Consider alternatives to fluticasone, particularly for long‑term use. May result in significantly reduced serum cortisol concentrations. Systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression have been reported during postmarketing use in patients receiving ritonavir and inhaled or intranasally administered fluticasone. Coadministration of fluticasone and LEXIVA/ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects. |
|
Lipid Modifying Agents: HMG-CoA reductase inhibitors: | ||
|
Atorvastatina |
↑Atorvastatin |
Titrate atorvastatin dose carefully and use the lowest necessary dose; do not exceed atorvastatin 20 mg/day. |
|
Lovastatin, simvastatin |
↑Lovastatin ↑Simvastatin |
Coadministration with lovastatin or simvastatin is contraindicated due to potential for serious reactions such as risk of myopathy including rhabdomyolysis [see Contraindications (4)]. |
|
Other lipid modifying agents: | ||
|
Lomitapide |
↑Lomitapide |
Coadministration with lomitapide is contraindicated due to potential for markedly increased transaminases. |
|
Narcotic analgesic: Methadone |
↓Methadone |
Data suggest that the interaction is not clinically relevant; however, patients should be monitored for opiate withdrawal symptoms. |
|
Fentanyl |
↑Fentanyl |
Careful monitoring of therapeutic effects and adverse effects of fentanyl (including potentially fatal respiratory depression) is recommended. |
|
Oral contraceptives: Ethinyl estradiol/ |
LEXIVA: ↓Amprenavir ↓Ethinyl estradiol LEXIVA/ritonavir: ↓Ethinyl estradiol |
Alternative methods of non-hormonal contraception are recommended. May lead to loss of virologic response.a Increased risk of transaminase elevations. No data are available on the use of LEXIVA/ritonavir with other hormonal therapies, such as hormone replacement therapy (HRT) for postmenopausal women. |
|
PDE5 inhibitors: Sildenafil, tadalafil, vardenafil |
↑Sildenafil ↑Tadalafil ↑Vardenafil |
May result in an increase in PDE5 inhibitor‑associated adverse events, including hypotension, syncope, visual disturbances, and priapism. Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH): • • Coadministration of ADCIRCA in patients on LEXIVA: Use of PDE5 inhibitors for erectile dysfunction: LEXIVA: Sildenafil: 25 mg every 48 hours. Tadalafil: no more than 10 mg every 72 hours. Vardenafil: no more than 2.5 mg every 24 hours. LEXIVA/ritonavir: Sildenafil: 25 mg every 48 hours. Tadalafil: no more than 10 mg every 72 hours. Vardenafil: no more than 2.5 mg every 72 hours. Use with increased monitoring for adverse events. |
|
Proton pump inhibitors: Esomeprazolea, lansoprazole, omeprazole, pantoprazole, rabeprazole |
LEXIVA: ↔Amprenavir ↑Esomeprazole LEXIVA/ritonavir: ↔Amprenavir ↔Esomeprazole |
Proton pump inhibitors can be administered at the same time as a dose of LEXIVA with no change in plasma amprenavir concentrations. |
|
Sedative/hypnotics: Midazolam, triazolam |
↑Midazolam ↑Triazolam |
Coadministration is contraindicated due to potential for serious and/or life‑threatening reactions such as prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
|
Tricyclic antidepressants: Amitriptyline, imipramine |
↑Tricyclics |
Therapeutic concentration monitoring is recommended for tricyclic antidepressants. |
•
Coadministration of LEXIVA with drugs that induce CYP3A4 may decrease amprenavir (active metabolite) concentrations leading to potential loss of virologic activity. (7, 12.3)
•
Coadministration with drugs that inhibit CYP3A4 may increase amprenavir concentrations. (7, 12.3)
•
Coadministration of LEXIVA or LEXIVA and ritonavir may result in clinically significant interactions with drugs metabolized by CYP3A4. (7)
•
Coadministration of LEXIVA and ritonavir may result in clinically significant interactions with drugs metabolized by CYP2D6. (7)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to LEXIVA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Limited data are available for use of LEXIVA in pregnancy. LEXIVA 700 mg twice daily taken with ritonavir 100 mg twice daily should only be considered in pregnant patients who are already on a stable twice-daily regimen of LEXIVA/ritonavir 700 mg/100 mg prior to pregnancy, and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) (see Clinical Considerations and Data).
There are insufficient human data on the use of fosamprenavir during pregnancy to adequately assess a drug-associated risk for birth defects and miscarriage. Given the limited number of pregnancies exposed to fosamprenavir-based regimens, no conclusions can be drawn on the safety of fosamprenavir in pregnancy. The background risk for major birth defects and miscarriage for the indicated population is unknown. The background rate for major birth defects in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) is 2.7% (see Data). The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15% to 20%.
In animal reproduction studies, no evidence of major adverse developmental outcomes was observed following oral administration of fosamprenavir. Systemic exposure to amprenavir (the active ingredient) was less than (rabbits) or up to 2 times (rats) those in humans at the maximum recommended human dose (MRHD) with or without ritonavir. In contrast, oral administration of amprenavir was associated with abortions in pregnant rabbits at doses that produced approximately one-twentieth the human exposure at the MRHD.
In the rat pre- and postnatal development study, toxicities to the offspring, including reduced survival and reproductive performance, were observed at maternal systemic exposures (AUC) to amprenavir that were approximately 2 times the exposure in humans at the MRHD of fosamprenavir alone or approximately the same as those seen in humans following administration of the MRHD of fosamprenavir in combination with ritonavir (see Data).
Clinical Considerations
Virologic Monitoring During Pregnancy and the Postpartum Period: Based on limited data on the use of LEXIVA during pregnancy, no dosage adjustments are required for pregnant patients who are already on a stable twice-daily regimen of LEXIVA 700 mg taken with ritonavir 100 mg prior to pregnancy, and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)]. In a clinical trial of 10 HIV-1–infected pregnant women treated with LEXIVA 700 mg taken with ritonavir 100 mg twice daily through postpartum, total amprenavir exposures were lower during pregnancy compared with the postpartum period. Therefore, viral load should be monitored closely to ensure viral suppression is maintained [see Data, Dosage and Administration (2.2), Clinical Pharmacology (12.3)]. Pregnancy data with other dosage regimens of LEXIVA (with or without ritonavir) are not available.
Data
Human Data: LEXIVA 700 mg taken with ritonavir 100 mg twice daily in combination with a background regimen was evaluated in a clinical trial of 10 HIV-1–infected pregnant women during the second and third trimesters and postpartum. Subjects initiated LEXIVA/ritonavir during pregnancy at a median of 19 weeks’ gestation; 4 had undetectable HIV-1 RNA viral load (less than 50 copies/mL) at the time of initiation. Amprenavir pharmacokinetics and placental transfer were studied during the second trimester (n = 6) or third trimester (n = 9) and postpartum (n = 9). Pregnancy outcomes were available for all 10 subjects, among which 1 twin pregnancy was included. Compared to the postpartum period, geometric mean amprenavir AUC was 35% lower in the second trimester and 25% lower in the third trimester. The amprenavir geometric mean ratio (95% CI) of fetal cord to maternal peripheral plasma concentration (n = 7) was 0.27 (0.24 to 0.30) [see Clinical Pharmacology (12.3)]. At delivery, 9 subjects had HIV-1 viral load less than 50 copies/mL, and 1 subject had a viral load of 111 copies/mL. All 11 infants born had test results that were negative for HIV-1 at the time of delivery and through 12 months postpartum. There were no new safety findings compared with the known safety profile of LEXIVA/ritonavir in HIV-1–infected adults.
Based on prospective reports to the APR of approximately 146 live births following exposure to fosamprenavir-containing regimens, there were 2 birth defects reported in 109 first trimester exposures and 2 birth defects reported in 36 second and third trimester exposures. The background rate for major birth defects is 2.7% in a U.S. reference population of the MACDP. Prospective reports from the APR of overall major birth defects in pregnancies exposed to fosamprenavir are compared with the U.S. background major birth defect rate. Methodological limitations of the APR include the use of the MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations as well as confounding due to the underlying disease.
Animal Data: Fosamprenavir was administered orally to pregnant rats (300, 820, or 2,240 mg per kg per day) and rabbits (74.8, 224.3, or 672.8 mg per kg per day) on Gestation Days 6 to 17 and Days 7 to 20, respectively. No major adverse effects on embryo-fetal development were observed at these dose levels, resulting in exposures (AUC0-24 h) approximately 2 times (rats) and 0.8 times (rabbits) human exposures at the MRHD of fosamprenavir alone or 0.7 times (rats) and 0.3 times (rabbits) human exposures at the MRHD of fosamprenavir in combination with ritonavir. However, increased incidence of abortion was observed in rabbits administered a maternally toxic dose of fosamprenavir (672.8 mg per kg per day). In a study where amprenavir was administered orally to pregnant rabbits (25, 50, or 100 mg per kg per day) on Gestation Days 8 to 20, increased abortions and an increased incidence of minor skeletal variations (deficient ossification of the femur, humerus, and trochlea) were observed at doses that produced approximately one-twentieth the exposure seen at the MRHD.
In the rat pre- and postnatal development study, fosamprenavir was administered orally (300, 820, or 2,240 mg per kg per day) on Gestation Day 6 to Lactation/Postpartum Day 20. Fosamprenavir caused a reduction in pup survival and body weights. In surviving female offspring from the high-dose group, an increased time to successful mating, an increased length of gestation, a reduced number of uterine implantation sites per litter, and reduced gestational body weights were observed. Systemic exposure (AUC0-24 h) to amprenavir in rats was approximately 2 times the exposures in humans at the MRHD of fosamprenavir alone or approximately the same as those seen in humans at the MRHD of fosamprenavir in combination with ritonavir.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommends that HIV-1–infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection.
There is no information available on the presence of amprenavir in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. When administered to lactating rats, amprenavir was present in milk (see Data). Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving LEXIVA.
Data
Amprenavir was excreted into the milk of lactating rats following a single dose of amprenavir (100 mg per kg); a maximal milk concentration was achieved 2 hours post-administration at a milk concentration approximately 1.2 times that of maternal plasma concentrations.
8.3 Females and Males of Reproductive Potential
Contraception
Use of LEXIVA may reduce the efficacy of combined hormonal contraceptives. Advise patients using combined hormonal contraceptives to use an effective alternative contraceptive method or an additional barrier method of contraception [see Drug Interactions (7.2)].
8.4 Pediatric Use
The safety, pharmacokinetic profile, and virologic and immunologic responses of LEXIVA with and without ritonavir were evaluated in protease inhibitor- naive and -experienced HIV-1–infected pediatric subjects aged at least 4 weeks to younger than 18 years and weighing at least 3 kg in 3 open-label trials [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.3)].
Treatment with LEXIVA is not recommended in protease inhibitor-experienced pediatric patients younger than 6 months. The pharmacokinetics, safety, tolerability, and efficacy of LEXIVA in pediatric patients younger than 4 weeks have not been established [see Clinical Pharmacology (12.3)]. Available pharmacokinetic and clinical data do not support once-daily dosing of LEXIVA alone or in combination with ritonavir for any pediatrics or twice-daily dosing without ritonavir in pediatric patients younger than 2 years [see Clinical Pharmacology (12.3), Clinical Studies (14.3)]. See Dosage and Administration (2.3) for dosing recommendations for pediatric patients.
8.5 Geriatric Use
Clinical studies of LEXIVA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger adults. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Amprenavir is principally metabolized by the liver; therefore, caution should be exercised when administering LEXIVA to patients with hepatic impairment because amprenavir concentrations may be increased [see Clinical Pharmacology (12.3)]. Patients with impaired hepatic function receiving LEXIVA with or without concurrent ritonavir require dose reduction [see Dosage and Administration (2.4)].
There are no data to support dosing recommendations for pediatric subjects with hepatic impairment.
Lactation: Breastfeeding is not recommended due to potential for HIV transmission. (8.2)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fosamprenavir is an HIV-1 antiretroviral agent [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetic properties of amprenavir after administration of LEXIVA, with or without ritonavir, have been evaluated in both healthy adult volunteers and in HIV-1–infected subjects; no substantial differences in steady-state amprenavir concentrations were observed between the 2 populations.
The pharmacokinetic parameters of amprenavir after administration of LEXIVA (with and without concomitant ritonavir) are shown in Table 7.
Table 7. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters in Adults|
a Data shown are median (range). | ||||
|
Regimen |
C****max (mcg/mL) |
T****max **(hours)**a |
AUC****24 (mcg•h/mL) |
Ctaub (mcg/mL) |
|
LEXIVA 1,400 mg Twice Daily (n = 22)c |
4.82 (4.06-5.72) |
1.3 (0.8-4.0) |
33.0d (27.6-39.2) |
0.35 (0.27-0.46) |
|
LEXIVA 1,400 mg Once Daily plus Ritonavir 200 mg Once Daily (n = 22)e |
7.24 (6.32-8.28) |
2.1 (0.8-5.0) |
69.4 (59.7-80.8) |
1.45 (1.16-1.81) |
|
LEXIVA 1,400 mg Once Daily plus Ritonavir 100 mg Once Daily (n = 36)e |
7.93 (7.25-8.68) |
1.5 (0.75-5.0) |
66.4 (61.1-72.1) |
0.86 (0.74-1.01) |
|
LEXIVA 700 mg Twice Daily plus Ritonavir 100 mg Twice Daily (n = 24)e |
6.08 (5.38-6.86) |
1.5 (0.75-5.0) |
79.2d (69.0-90.6) |
2.12 (1.77-2.54) |
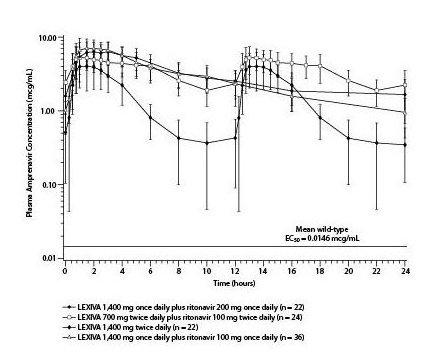
The mean plasma amprenavir concentrations of the dosing regimens over the dosing intervals are displayed in Figure 1.
Figure 1. Mean (±SD) Steady-State Plasma Amprenavir Concentrations and Mean EC50 Values against HIV from Protease Inhibitor-Naive Subjects (in the Absence of Human Serum)
Absorption
After administration of a single dose of LEXIVA to HIV-1–infected subjects, the time to peak amprenavir concentration (Tmax) occurred between 1.5 and 4 hours (median 2.5 hours). The absolute oral bioavailability of amprenavir after administration of LEXIVA in humans has not been established.
After administration of a single 1,400-mg dose in the fasted state, LEXIVA oral suspension (50 mg per mL) and LEXIVA tablets (700 mg) provided similar amprenavir exposures (AUC); however, the Cmax of amprenavir after administration of the suspension formulation was 14.5% higher compared with the tablet.
Amprenavir is both a substrate for and inducer of P-glycoprotein.
Effects of Food on Oral Absorption
Administration of a single 1,400-mg dose of LEXIVA tablets in the fed state (standardized high-fat meal: 967 kcal, 67 grams fat, 33 grams protein, 58 grams carbohydrate) compared with the fasted state was associated with no significant changes in amprenavir Cmax, Tmax, or AUC0-∞ [see Dosage and Administration (2)].
Administration of a single 1,400-mg dose of LEXIVA oral suspension in the fed state (standardized high-fat meal: 967 kcal, 67 grams fat, 33 grams protein, 58 grams carbohydrate) compared with the fasted state was associated with a 46% reduction in Cmax, a 0.72-hour delay in Tmax, and a 28% reduction in amprenavir AUC0-∞.
Distribution
In vitro, amprenavir is approximately 90% bound to plasma proteins, primarily to alpha1-acid glycoprotein. In vitro, concentration-dependent binding was observed over the concentration range of 1 to 10 mcg per mL, with decreased binding at higher concentrations. The partitioning of amprenavir into erythrocytes is low, but increases as amprenavir concentrations increase, reflecting the higher amount of unbound drug at higher concentrations.
Metabolism
After oral administration, fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate prior to reaching the systemic circulation. This occurs in the gut epithelium during absorption. Amprenavir is metabolized in the liver by the CYP3A4 enzyme system. The 2 major metabolites result from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
Elimination
Excretion of unchanged amprenavir in urine and feces is minimal. Unchanged amprenavir in urine accounts for approximately 1% of the dose; unchanged amprenavir was not detectable in feces. Approximately 14% and 75% of an administered single dose of 14C-amprenavir can be accounted for as metabolites in urine and feces, respectively. Two metabolites accounted for greater than 90% of the radiocarbon in fecal samples. The plasma elimination half-life of amprenavir is approximately 7.7 hours.
Specific Populations
Patients with Hepatic Impairment: The pharmacokinetics of amprenavir have been studied after the administration of LEXIVA in combination with ritonavir to adult HIV-1–infected subjects with mild, moderate, and severe hepatic impairment. Following 2 weeks of dosing with LEXIVA plus ritonavir, the AUC of amprenavir was increased by approximately 22% in subjects with mild hepatic impairment, by approximately 70% in subjects with moderate hepatic impairment, and by approximately 80% in subjects with severe hepatic impairment compared with HIV-1–infected subjects with normal hepatic function. Protein binding of amprenavir was decreased in subjects with hepatic impairment. The unbound fraction at 2 hours (approximate Cmax) ranged between a decrease of -7% to an increase of 57% while the unbound fraction at the end of the dosing interval (Cmin) increased from 50% to 102% [see Dosage and Administration (2.4)].
The pharmacokinetics of amprenavir have been studied after administration of amprenavir given as AGENERASE capsules to adult subjects with hepatic impairment. Following administration of a single 600-mg oral dose, the AUC of amprenavir was increased by approximately 2.5-fold in subjects with moderate cirrhosis and by approximately 4.5-fold in subjects with severe cirrhosis compared with healthy volunteers [see Dosage and Administration (2.4)].
Patients with Renal Impairment: The impact of renal impairment on amprenavir elimination in adults has not been studied. The renal elimination of unchanged amprenavir represents approximately 1% of the administered dose; therefore, renal impairment is not expected to significantly impact the elimination of amprenavir.
Pregnant Women: Amprenavir pharmacokinetics were studied in pregnant women receiving LEXIVA (700 mg) plus ritonavir (100 mg) twice daily during the second trimester (n = 6) or third trimester (n = 9) and postpartum (n = 9). Compared to postpartum, geometric mean amprenavir AUC was 35% lower in the second trimester and 25% lower in the third trimester (Table 8). This decrease results in amprenavir concentrations that are within the range observed across regimens of LEXIVA in non-pregnant adults and lower concentrations compared with LEXIVA (700 mg) plus ritonavir (100 mg) twice daily in non-pregnant adults (Table 7, Table 8). This decrease is not expected to be considered clinically relevant in patients who are virologically suppressed; however, viral load should be monitored closely to ensure viral suppression is maintained [see Dosage and Administration (2.2), Use in Specific Populations (8.1)]. The amprenavir geometric mean ratio (95% CI) of fetal cord to maternal peripheral plasma concentration (n = 7) was 0.27 (0.24 to 0.30).
Table 8. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters in Pregnant Women Receiving LEXIVA with Ritonavir|
a AUC24 was calculated from AUC12 summary data x 2. | |||
|
Pharmacokinetic Parameter |
LEXIVA 700 mg Twice Daily plus Ritonavir 100 mg Twice Daily | ||
|
Second Trimester (n = 6) |
Third Trimester (n = 9) |
Postpartum (n = 9) | |
|
AUC12 (mcg•h/mL) |
26.0 (19.5, 34.6) |
30.1 (21.6, 41.9) |
39.9 (31.9, 50.1) |
|
AUC24 (mcg•h/mL)a |
52.0 (39.0, 69.2) |
60.2 (43.2, 83.8) |
79.8 (63.8, 100.2) |
|
Cmax (mcg/mL) |
4.19 (3.19, 5.51) |
5.36 (3.98, 7.22) |
6.65 (5.24, 8.44) |
|
Ctau (mcg/mL)b |
1.31 (0.97, 1.77) |
1.34 (0.95, 1.89) |
2.03 (1.46, 2.83) |
Pediatric Patients: The pharmacokinetics of amprenavir following administration of LEXIVA oral suspension and LEXIVA tablets, with or without ritonavir, have been studied in a total of 212 HIV-1–infected pediatric subjects enrolled in 3 trials. LEXIVA without ritonavir was administered as 30 or 40 mg per kg twice daily to children aged 2 to 5 years. LEXIVA with ritonavir was administered as LEXIVA 30 mg per kg plus ritonavir 6 mg per kg once daily to children aged 2 to 18 years and as LEXIVA 18 to 60 mg per kg plus ritonavir 3 to 10 mg per kg twice daily to children aged at least 4 weeks to 18 years; body weights ranged from 3 to 103 kg.
Amprenavir apparent clearance decreased with increasing weight. Weight- adjusted apparent clearance was higher in children younger than 4 years, suggesting that younger children require higher mg-per-kg dosing of LEXIVA.
The pharmacokinetics of LEXIVA oral suspension in protease inhibitor-naive infants younger than 6 months (n = 9) receiving LEXIVA 45 mg per kg plus ritonavir 10 mg per kg twice daily generally demonstrated lower AUC12 and Cmin than adults receiving twice-daily LEXIVA 700 mg plus ritonavir 100 mg, the dose recommended for protease-experienced adults. The mean steady-state amprenavir AUC12, Cmax, and Cmin were 26.6 mcg•hour per mL, 6.25 mcg per mL, and 0.86 mcg per mL, respectively. Because of expected low amprenavir exposure and a requirement for large volume of drug, twice-daily dosing of LEXIVA alone (without ritonavir) in pediatric subjects younger than 2 years was not studied.
Pharmacokinetic parameters for LEXIVA administered with food and with ritonavir in this patient population at the recommended weight-band–based dosage regimens are provided in Table 9.
Table 9. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters by Weight in Pediatric and Adolescent Subjects Aged at Least 4 Weeks to 18 Years Receiving LEXIVA with Ritonavir|
a Recommended dose for pediatric patients weighing 11 kg to less than 15 kg is based on population pharmacokinetic analysis. | |||||||
|
Weight |
Recommended Dosage Regimen |
C****max |
AUC****24 |
C****min | |||
|
n |
(mcg/mL) |
n |
(mcg•h/mL) |
n |
(mcg/mL) | ||
|
<11 kg |
LEXIVA 45 mg/kg plus Ritonavir 7 mg/kg twice daily |
12 |
6.00 (3.88, 9.29) |
12 |
57.3 (34.1, 96.2) |
27 |
1.65 (1.22, 2.24) |
|
11 kg - <15 kg |
LEXIVA 30 mg/kg plus Ritonavir 3 mg/kg twice daily |
Not studieda | |||||
|
15 kg - <20 kg |
LEXIVA 23 mg/kg plus Ritonavir 3 mg/kg twice daily |
5 |
9.54 (4.63, 19.7) |
5 |
121 (54.2, 269) |
9 |
3.56 (2.33, 5.43) |
|
20 kg - <39 kg |
LEXIVA 18 mg/kg plus Ritonavir 3 mg/kg twice daily |
13 |
6.24 (5.01, 7.77) |
12 |
97.9 (77.0, 124) |
23 |
2.54 (2.11, 3.06) |
|
≥39 kg |
LEXIVA 700 mg plus Ritonavir 100 mg twice daily |
15 |
5.03 (4.04, 6.26) |
15 |
72.3 (59.6, 87.6) |
42 |
1.98 (1.72, 2.29) |
Subjects aged 2 to younger than 6 years receiving LEXIVA 30 mg per kg twice daily without ritonavir achieved geometric mean (95% CI) amprenavir Cmax (n = 9), AUC12 (n = 9), and Cmin (n = 19) of 7.15 (5.05, 10.1), 22.3 (15.3, 32.6), and 0.513 (0.384, 0.686), respectively.
Geriatric Patients: The pharmacokinetics of amprenavir after administration of LEXIVA to patients older than 65 years have not been studied [see Use in Specific Populations (8.5)].
Male and Female Patients: The pharmacokinetics of amprenavir after administration of LEXIVA do not differ between males and females.
Racial Groups: The pharmacokinetics of amprenavir after administration of LEXIVA do not differ between blacks and non-blacks.
Drug Interaction Studies
[See Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7).]
Amprenavir, the active metabolite of fosamprenavir, is metabolized in the liver by the cytochrome P450 enzyme system. Amprenavir inhibits CYP3A4. Data also suggest that amprenavir induces CYP3A4. Caution should be used when coadministering medications that are substrates, inhibitors, or inducers of CYP3A4, or potentially toxic medications that are metabolized by CYP3A4. Amprenavir does not inhibit CYP2D6, CYP1A2, CYP2C9, CYP2C19, CYP2E1, or uridine glucuronosyltransferase (UDPGT). Amprenavir is both a substrate for and inducer of P-glycoprotein.
Drug interaction trials were performed with LEXIVA and other drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration on AUC, Cmax, and Cmin values are summarized in Table 10 (effect of other drugs on amprenavir) and Table 12 (effect of LEXIVA on other drugs). In addition, since LEXIVA delivers comparable amprenavir plasma concentrations as AGENERASE, drug interaction data derived from trials with AGENERASE are provided in Tables 11 and 13. For information regarding clinical recommendations, [see Drug Interactions (7)].
Table 10. Drug Interactions: Pharmacokinetic Parameters for Amprenavir after Administration of LEXIVA in the Presence of the Coadministered Drug(s)|
a Concomitant medication is also shown in this column where appropriate. | |||||
|
Coadministered Drug(s) and Dose(s) |
Dose of LEXIVA****a |
n |
% Change in Amprenavir Pharmacokinetic Parameters (90% CI) | ||
|
C****max |
AUC |
C****min | |||
|
Antacid (MAALOX TC) 30-mL single dose |
1,400-mg single dose |
30 |
↓35 (↓24 to ↓42) |
↓18 (↓9 to ↓26) |
↑14 (↓7 to ↑39) |
|
Atazanavir 300 mg once daily for 10 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days |
22 |
↔ |
↔ |
↔ |
|
Atorvastatin 10 mg once daily for 4 days |
1,400 mg twice daily for 2 weeks |
16 |
↓18 (↓34 to ↑1) |
↓27 (↓41 to ↓12) |
↓12 (↓27 to ↓6) |
|
Atorvastatin 10 mg once daily for 4 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
16 |
↔ |
↔ |
↔ |
|
Efavirenz 600 mg once daily for 2 weeks |
1,400 mg once daily plus ritonavir 200 mg once daily for 2 weeks |
16 |
↔ |
↓13 (↓30 to ↑7) |
↓36 (↓8 to ↓56) |
|
Efavirenz 600 mg once daily plus additional ritonavir 100 mg once daily for 2 weeks |
1,400 mg once daily plus ritonavir 200 mg once daily for 2 weeks |
16 |
↑18 (↑1 to ↑38) |
↑11 (0 to ↑24) |
↔ |
|
Efavirenz 600 mg once daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
16 |
↔ |
↔ |
↓17 (↓4 to ↓29) |
|
Esomeprazole 20 mg once daily for 2 weeks |
1,400 mg twice daily for 2 weeks |
25 |
↔ |
↔ |
↔ |
|
Esomeprazole 20 mg once daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
23 |
↔ |
↔ |
↔ |
|
Ethinyl estradiol/ 0.035 mg/0.5 mg once daily for 21 days |
700 mg twice daily plus ritonavirb 100 mg twice daily for 21 days |
25 |
↔c |
↔c |
↔c |
|
Ketoconazoled 200 mg once daily for 4 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 4 days |
15 |
↔ |
↔ |
↔ |
|
Lopinavir/ritonavir 533 mg/133 mg twice daily |
1,400 mg twice daily for 2 weeks |
18 |
↓13e |
↓26e |
↓42e |
|
Lopinavir/ritonavir 400 mg/100 mg twice daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
18 |
↓58 (↓42 to ↓70) |
↓63 (↓51 to ↓72) |
↓65 (↓54 to ↓73) |
|
Maraviroc 300 mg twice daily for 10 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 20 days |
14 |
↓34 (↓25 to ↓41) |
↓35 (↓29 to ↓41) |
↓36 (↓27 to ↓43) |
|
Maraviroc 300 mg once daily for 10 days |
1,400 mg once daily plus ritonavir 100 mg once daily for 20 days |
14 |
↓29 (↓20 to ↓38) |
↓30 (↓23 to ↓36) |
↓15 (↓3 to ↓25) |
|
Methadone 70 to 120 mg once daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
19 |
↔c |
↔c |
↔c |
|
Nevirapine 200 mg twice daily for 2 weeksf |
1,400 mg twice daily for 2 weeks |
17 |
↓25 (↓37 to ↓10) |
↓33 (↓45 to ↓20) |
↓35 (↓50 to ↓15) |
|
Nevirapine 200 mg twice daily for 2 weeksf |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
17 |
↔ |
↓11 (↓23 to ↑3) |
↓19 (↓32 to ↓4) |
|
Phenytoin 300 mg once daily for 10 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days |
13 |
↔ |
↑20 (↑8 to ↑34) |
↑19 (↑6 to ↑33) |
|
Raltegravir 400 mg twice daily for 14 days |
1,400 mg twice daily for 14 days (fasted) |
14 |
↓27 (↓46 to ↔) |
↓36 (↓53 to ↓13) |
↓43g (↓59 to ↓21) |
|
1,400 mg twice daily for 14 daysh |
14 |
↓15 (↓27 to ↓1) |
↓17 (↓27 to ↓6) |
↓32g (↓53 to ↓1) | |
|
700 mg twice daily plus ritonavir 100 mg twice daily for 14 days (fasted) |
14 |
↓14 (↓39 to ↑20) |
↓17 (↓38 to ↑12) |
↓20g (↓45 to ↑17) | |
|
700 mg twice daily plus ritonavir 100 mg twice daily for 14 daysh |
12 |
↓25 (↓42 to ↓2) |
↓25 (↓44 to ↔) |
↓33g (↓52 to ↓7) | |
|
Raltegravir 400 mg twice daily for 14 days |
1,400 mg once daily plus ritonavir 100 mg once daily for 14 days (fasted) |
13 |
↓18 (↓34 to ↔) |
↓24 (↓41 to ↔) |
↓50g (↓64 to ↓31) |
|
1,400 mg once daily plus ritonavir 100 mg once daily for 14 daysh |
14 |
↑27 (↓1 to ↑62) |
↑13 (↓7 to ↑38) |
↓17g (↓45 to ↑26) | |
|
Ranitidine 300-mg single dose (administered 1 hour before fosamprenavir) |
1,400-mg single dose |
30 |
↓51 (↓43 to ↓58) |
↓30 (↓22 to ↓37) |
↔ (↓19 to ↑21) |
|
Rifabutin 150 mg every other day for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
15 |
↑36c (↑18 to ↑55) |
↑35c (↑17 to ↑56) |
↑17c (↓1 to ↑39) |
|
Tenofovir 300 mg once daily for 4 to 48 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 4 to 48 weeks |
45 |
NA |
NA |
↔i |
|
Tenofovir 300 mg once daily for 4 to 48 weeks |
1,400 mg once daily plus ritonavir 200 mg once daily for 4 to 48 weeks |
60 |
NA |
NA |
↔i |
|
a Compared with parallel control group. | |||||
|
Coadministered Drug(s) and Dose(s) |
Dose of AGENERASE****a |
n |
% Change in Amprenavir Pharmacokinetic Parameters (90% CI) | ||
|
C****max |
AUC |
C****min | |||
|
Abacavir 300 mg twice daily for 2 to 3 weeks |
900 mg twice daily for 2 to 3 weeks |
4 |
↔a |
↔a |
↔a |
|
Clarithromycin 500 mg twice daily for 4 days |
1,200 mg twice daily for 4 days |
12 |
↑15 (↑1 to ↑31) |
↑18 (↑8 to ↑29) |
↑39 (↑31 to ↑47) |
|
Delavirdine 600 mg twice daily for 10 days |
600 mg twice daily for 10 days |
9 |
↑40b |
↑130b |
↑125b |
|
Ethinyl estradiol/ norethindrone 0.035 mg/1 mg for 1 cycle |
1,200 mg twice daily for 28 days |
10 |
↔ |
↓22 (↓35 to ↓8) |
↓20 (↓41 to ↑8) |
|
Indinavir 800 mg 3 times a day for 2 weeks (fasted) |
750 or 800 mg 3 times a day for 2 weeks (fasted) |
9 |
↑18 (↑13 to ↑58) |
↑33 (↑2 to ↑73) |
↑25 (↓27 to ↑116) |
|
Ketoconazole 400-mg single dose |
1,200-mg single dose |
12 |
↓16 (↓25 to ↓6) |
↑31 (↑20 to ↑42) |
NA |
|
Lamivudine 150-mg single dose |
600-mg single dose |
11 |
↔ |
↔ |
NA |
|
Methadone 44 to 100 mg once daily for >30 days |
1,200 mg twice daily for 10 days |
16 |
↓27c |
↓30c |
↓25c |
|
Nelfinavir 750 mg 3 times a day for 2 weeks (fed) |
750 or 800 mg 3 times a day for 2 weeks (fed) |
6 |
↓14 (↓38 to ↑20) |
↔ |
↑189 (↑52 to ↑448) |
|
Rifabutin 300 mg once daily for 10 days |
1,200 mg twice daily for 10 days |
5 |
↔ |
↓15 (↓28 to 0) |
↓15 (↓38 to ↑17) |
|
Rifampin 300 mg once daily for 4 days |
1,200 mg twice daily for 4 days |
11 |
↓70 (↓76 to ↓62) |
↓82 (↓84 to ↓78) |
↓92 (↓95 to ↓89) |
|
Saquinavir 800 mg 3 times a day for 2 weeks (fed) |
750 or 800 mg 3 times a day for 2 weeks (fed) |
7 |
↓37 (↓54 to ↓14) |
↓32 (↓49 to ↓9) |
↓14 (↓52 to ↑54) |
|
Zidovudine 300-mg single dose |
600-mg single dose |
12 |
↔ |
↑13 (↓2 to ↑31) |
NA |
|
a Concomitant medication is also shown in this column where appropriate. | |||||
|
Coadministered Drug(s) and Dose(s) |
Dose of LEXIVA****a |
n |
% Change in Pharmacokinetic Parameters | ||
|
C****max |
AUC |
C****min | |||
|
Atazanavir 300 mg once daily for 10 daysb |
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days |
21 |
↓24 (↓39 to ↓6) |
↓22 (↓34 to ↓9) |
↔ |
|
Atorvastatin 10 mg once daily for 4 days |
1,400 mg twice daily for 2 weeks |
16 |
↑304 (↑205 to ↑437) |
↑130 (↑100 to ↑164) |
↓10 (↓27 to ↑12) |
|
Atorvastatin 10 mg once daily for 4 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
16 |
↑184 (↑126 to ↑257) |
↑153 (↑115 to ↑199) |
↑73 (↑45 to ↑108) |
|
Esomeprazole 20 mg once daily for 2 weeks |
1,400 mg twice daily for 2 weeks |
25 |
↔ |
↑55 (↑39 to ↑73) |
ND |
|
Esomeprazole 20 mg once daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
23 |
↔ |
↔ |
ND |
|
Ethinyl estradiolc 0.035 mg once daily for 21 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 21 days |
25 |
↓28 (↓21 to ↓35) |
↓37 (↓30 to ↓42) |
ND |
|
Dolutegravir 50 mg once daily |
700 mg twice daily plus ritonavir 100 mg twice daily |
12 |
↓24 (↓8 to ↓37) |
↓35 (↓22 to ↓46) |
↓49 (↓37 to ↓59) |
|
Ketoconazoled 200 mg once daily for 4 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 4 days |
15 |
↑25 (↑0 to ↑56) |
↑169 (↑108 to ↑248) |
ND |
|
Lopinavir/ritonavire 533 mg/133 mg twice daily for 2 weeks |
1,400 mg twice daily for 2 weeks |
18 |
↔f |
↔f |
↔f |
|
Lopinavir/ritonavire 400 mg/100 mg twice daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
18 |
↑30 (↓15 to ↑47) |
↑37 (↓20 to ↑55) |
↑52 (↓28 to ↑82) |
|
Maraviroc 300 mg twice daily for 10 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 20 days |
14 |
↑52 (↑27 to ↑82) |
↑149 (↑119 to ↑182) |
↑374 (↑303 to ↑457) |
|
Maraviroc 300 mg once daily for 10 days |
1,400 mg once daily plus ritonavir 100 mg once daily for 20 days |
14 |
↑45 (↑20 to ↑74) |
↑126 (↑99 to ↑158) |
↑80 (↑53 to ↑113) |
|
Methadone 70 to 120 mg once daily for 2 weeks |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
19 |
R-Methadone (active) | ||
|
↓21g (↓30 to ↓12) |
↓18g (↓27 to ↓8) |
↓11g (↓21 to ↑1) | |||
|
S-Methadone (inactive) | |||||
|
↓43g (↓49 to ↓37) |
↓43g (↓50 to ↓36) |
↓41g (↓49 to ↓31) | |||
|
Nevirapine 200 mg twice daily for 2 weeksh |
1,400 mg twice daily for 2 weeks |
17 |
↑25 (↑14 to ↑37) |
↑29 (↑19 to ↑40) |
↑34 (↑20 to ↑49) |
|
Nevirapine 200 mg twice daily for 2 weeksh |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
17 |
↑13 (↑3 to ↑24) |
↑14 (↑5 to ↑24) |
↑22 (↑9 to ↑35) |
|
Norethindronec 0.5 mg once daily for 21 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 21 days |
25 |
↓38 (↓32 to ↓44) |
↓34 (↓30 to ↓37) |
↓26 (↓20 to ↓32) |
|
Phenytoin 300 mg once daily for 10 days |
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days |
14 |
↓20 (↓12 to ↓27) |
↓22 (↓17 to ↓27) |
↓29 (↓23 to ↓34) |
|
Rifabutin 150 mg every other day for 2 weeks i |
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks |
15 |
↓14 (↓28 to ↑4) |
↔ |
↑28 (↑12 to ↑46) |
|
(25-O-desacetylrifabutin metabolite) |
↑579 (↑479 to ↑698) |
↑1,120 (↑965 to ↑1,300) |
↑2,510 (↑1,910 to ↑3,300) | ||
|
Rifabutin + 25-O- desacetylrifabutin metabolite |
NA |
↑64 (↑46 to ↑84) |
NA | ||
|
Rosuvastatin 10-mg single dose |
700 mg twice daily plus ritonavir 100 mg twice daily for 7 days |
(↑45) |
(↑8) |
NA |
|
a Compared with historical data. | |||||
|
Coadministered |
Dose of AGENERASE |
n |
% Change in Pharmacokinetic Parameters of Coadministered Drug (90% CI) | ||
|
C****max |
AUC |
C****min | |||
|
Abacavir 300 mg twice daily for 2 to 3 weeks |
900 mg twice daily for 2 to 3 weeks |
4 |
↔a |
↔a |
↔a |
|
Clarithromycin 500 mg twice daily for 4 days |
1,200 mg twice daily for 4 days |
12 |
↓10 (↓24 to ↑7) |
↔ |
↔ |
|
Delavirdine 600 mg twice daily for 10 days |
600 mg twice daily for 10 days |
9 |
↓47b |
↓61b |
↓88b |
|
Ethinyl estradiol 0.035 mg for 1 cycle |
1,200 mg twice daily for 28 days |
10 |
↔ |
↔ |
↑32 (↓3 to ↑79) |
|
Indinavir 800 mg 3 times a day for 2 weeks (fasted) |
750 mg or 800 mg 3 times a day for 2 weeks (fasted) |
9 |
↓22a |
↓38a |
↓27a |
|
Ketoconazole 400-mg single dose |
1,200-mg single dose |
12 |
↑19 (↑8 to ↑33) |
↑44 (↑31 to ↑59) |
NA |
|
Lamivudine 150-mg single dose |
600-mg single dose |
11 |
↔ |
↔ |
NA |
|
Methadone 44 to 100 mg once daily for >30 days |
1,200 mg twice daily for 10 days |
16 |
R-Methadone (active) | ||
|
↓25 (↓32 to ↓18) |
↓13 (↓21 to ↓5) |
↓21 (↓32 to ↓9) | |||
|
S-Methadone (inactive) | |||||
|
↓48 (↓55 to ↓40) |
↓40 (↓46 to ↓32) |
↓53 (↓60 to ↓43) | |||
|
Nelfinavir 750 mg 3 times a day for 2 weeks (fed) |
750 mg or 800 mg 3 times a day for 2 weeks (fed) |
6 |
↑12a |
↑15a |
↑14a |
|
Norethindrone 1 mg for 1 cycle |
1,200 mg twice daily for 28 days |
10 |
↔ |
↑18 (↑1 to ↑38) |
↑45 (↑13 to ↑88) |
|
Rifabutin 300 mg once daily for 10 days |
1,200 mg twice daily for 10 days |
5 |
↑119 (↑82 to ↑164) |
↑193 (↑156 to ↑235) |
↑271 (↑171 to ↑409) |
|
Rifampin 300 mg once daily for 4 days |
1,200 mg twice daily for 4 days |
11 |
↔ |
↔ |
ND |
|
Saquinavir 800 mg 3 times a day for 2 weeks (fed) |
750 mg or 800 mg 3 times a day for 2 weeks (fed) |
7 |
↑21a |
↓19a |
↓48a |
|
Zidovudine 300-mg single dose |
600-mg single dose |
12 |
↑40 (↑14 to ↑71) |
↑31 (↑19 to ↑45) |
NA |
12.4 Microbiology
Mechanism of Action
Fosamprenavir is a prodrug that is rapidly hydrolyzed to amprenavir by cellular phosphatases in the gut epithelium as it is absorbed. Amprenavir is an inhibitor of HIV-1 protease. Amprenavir binds to the active site of HIV-1 protease and thereby prevents the processing of viral Gag and Gag-Pol polyprotein precursors, resulting in the formation of immature non-infectious viral particles.
Antiviral Activity
Fosamprenavir has little or no antiviral activity in cell culture. The antiviral activity of amprenavir was evaluated against HIV-1 IIIB in both acutely and chronically infected lymphoblastic cell lines (MT-4, CEM-CCRF, H9) and in peripheral blood lymphocytes in cell culture. The 50% effective concentration (EC50) of amprenavir ranged from 0.012 to 0.08 microM in acutely infected cells and was 0.41 microM in chronically infected cells (1 microM = 0.50 mcg per mL). The median EC50 value of amprenavir against HIV-1 isolates from clades A to G was 0.00095 microM in peripheral blood mononuclear cells (PBMCs). Similarly, the EC50 values for amprenavir against monocytes/macrophage tropic HIV-1 isolates (clade B) ranged from 0.003 to 0.075 microM in monocyte/macrophage cultures. The EC50 values of amprenavir against HIV-2 isolates grown in PBMCs were higher than those for HIV-1 isolates, and ranged from 0.003 to 0.11 microM. The anti-HIV-1 activity of amprenavir was not antagonistic in combination with the nucleoside reverse transcriptase inhibitors (NRTIs); abacavir, didanosine, lamivudine, stavudine, tenofovir, and zidovudine; the non-nucleoside reverse transcriptase inhibitors (NNRTIs) delavirdine, efavirenz, and nevirapine; the protease inhibitors (PIs) atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir; and the gp41 fusion inhibitor enfuvirtide. These drug combinations have not been adequately studied in humans.
Resistance
HIV-1 isolates with decreased susceptibility to amprenavir have been selected in cell culture and obtained from subjects treated with fosamprenavir. Genotypic analysis of isolates from treatment-naive subjects failing amprenavir-containing regimens showed substitutions in the HIV-1 protease resulting in amino acid substitutions primarily at positions V32I, M46I/L, I47V, I50V, I54L/M, and I84V, as well as substitutions in the p7/p1 and p1/p6 Gag and Gag-Pol polyprotein precursor cleavage sites. Some of these amprenavir resistance-associated substitutions have also been detected in HIV-1 isolates from antiretroviral-naive subjects treated with LEXIVA. Of the 488 antiretroviral-naive subjects treated with LEXIVA 1,400 mg twice daily or LEXIVA 1,400 mg plus ritonavir 200 mg once daily in Trials APV30001 and APV30002, respectively, isolates from 61 subjects (29 receiving LEXIVA and 32 receiving LEXIVA/ritonavir) with virologic failure (plasma HIV-1 RNA greater than 1,000 copies per mL on 2 occasions on or after Week 12) were genotyped. Isolates from 5 of the 29 antiretroviral-naive subjects (17%) receiving LEXIVA without ritonavir in Trial APV30001 had evidence of genotypic resistance to amprenavir: I54L/M (n = 2), I54L + L33F (n = 1), V32I + I47V (n = 1), and M46I
- I47V (n = 1). No amprenavir resistance-associated substitutions were detected in isolates from antiretroviral-naive subjects treated with LEXIVA/ritonavir for 48 weeks in Trial APV30002. However, the M46I and I50V substitutions were detected in isolates from 1 virologic failure subject receiving LEXIVA/ritonavir once daily at Week 160 (HIV-1 RNA greater than 500 copies per mL). Upon retrospective analysis of stored samples using an ultrasensitive assay, these resistant substitutions were traced back to Week 84 (76 weeks prior to clinical virologic failure).
Cross-Resistance
Varying degrees of cross-resistance among HIV-1 protease inhibitors have been observed. An association between virologic response at 48 weeks (HIV-1 RNA level less than 400 copies per mL) and protease inhibitor-resistance substitutions detected in baseline HIV-1 isolates from protease inhibitor- experienced subjects receiving LEXIVA/ritonavir twice daily (n = 88), or lopinavir/ritonavir twice daily (n = 85) in Trial APV30003 is shown in Table 14. The majority of subjects had previously received either one (47%) or 2 protease inhibitors (36%), most commonly nelfinavir (57%) and indinavir (53%). Out of 102 subjects with baseline phenotypes receiving twice-daily LEXIVA/ritonavir, 54% (n = 55) had resistance to at least one protease inhibitor, with 98% (n = 54) of those having resistance to nelfinavir. Out of 97 subjects with baseline phenotypes in the lopinavir/ritonavir arm, 60% (n = 58) had resistance to at least one protease inhibitor, with 97% (n = 56) of those having resistance to nelfinavir.
Table 14. Responders at Trial Week 48 by Presence of Baseline Protease Inhibitor Resistance-Associated Substitutionsa|
a Results should be interpreted with caution because the subgroups were small. | ||||
|
Protease Inhibitor |
LEXIVA/Ritonavir (n = 88) |
Lopinavir/Ritonavir (n = 85) | ||
|
D30N |
21/22 |
95% |
17/19 |
89% |
|
N88D/S |
20/22 |
91% |
12/12 |
100% |
|
L90M |
16/31 |
52% |
17/29 |
59% |
|
M46I/L |
11/22 |
50% |
12/24 |
50% |
|
V82A/F/T/S |
2/9 |
22% |
6/17 |
35% |
|
I54V |
2/11 |
18% |
6/11 |
55% |
|
I84V |
1/6 |
17% |
2/5 |
40% |
The virologic response based upon baseline phenotype was assessed. Baseline isolates from protease inhibitor-experienced subjects responding to LEXIVA/ritonavir twice daily had a median shift in susceptibility to amprenavir relative to a standard wild-type reference strain of 0.7 (range: 0.1 to 5.4, n = 62), and baseline isolates from individuals failing therapy had a median shift in susceptibility of 1.9 (range: 0.2 to 14, n = 29). Because this was a select patient population, these data do not constitute definitive clinical susceptibility break points. Additional data are needed to determine clinically relevant break points for LEXIVA.
Isolates from 15 of the 20 subjects receiving twice-daily LEXIVA/ritonavir up to Week 48 and experiencing virologic failure/ongoing replication were subjected to genotypic analysis. The following amprenavir resistance- associated substitutions were found either alone or in combination: V32I, M46I/L, I47V, I50V, I54L/M, and I84V. Isolates from 4 of the 16 subjects continuing to receive twice-daily LEXIVA/ritonavir up to Week 96 who experienced virologic failure underwent genotypic analysis. Isolates from 2 subjects contained amprenavir resistance-associated substitutions: V32I, M46I, and I47V in 1 isolate and I84V in the other.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In long-term carcinogenicity studies, fosamprenavir was administered orally for up to 104 weeks at doses of 250, 400, or 600 mg per kg per day in mice and at doses of 300, 825, or 2,250 mg per kg per day in rats. Exposures at these doses were 0.3- to 0.7-fold (mice) and 0.7- to 1.4-fold (rats) those in humans given 1,400 mg twice daily of fosamprenavir alone, and 0.2- to 0.3-fold (mice) and 0.3- to 0.7-fold (rats) those in humans given 1,400 mg once daily of fosamprenavir plus 200 mg ritonavir once daily. Exposures in the carcinogenicity studies were 0.1- to 0.3-fold (mice) and 0.3- to 0.6-fold (rats) those in humans given 700 mg of fosamprenavir plus 100 mg ritonavir twice daily. There was an increase in hepatocellular adenomas and hepatocellular carcinomas at all doses in male mice and at 600 mg per kg per day in female mice, and in hepatocellular adenomas and thyroid follicular cell adenomas at all doses in male rats, and at 825 mg per kg per day and 2,250 mg per kg per day in female rats. The relevance of the hepatocellular findings in the rodents for humans is uncertain. Repeat-dose studies with fosamprenavir in rats produced effects consistent with enzyme induction, which predisposes rats, but not humans, to thyroid neoplasms. In addition, in rats only there was an increase in interstitial cell hyperplasia at 825 mg per kg per day and 2,250 mg per kg per day, and an increase in uterine endometrial adenocarcinoma at 2,250 mg per kg per day. The incidence of endometrial findings was slightly increased over concurrent controls, but was within background range for female rats. The relevance of the uterine endometrial adenocarcinoma findings in rats for humans is uncertain.
Fosamprenavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays. These assays included bacterial reverse mutation (Ames), mouse lymphoma, rat micronucleus, and chromosome aberrations in human lymphocytes.
The effects of fosamprenavir on fertility and general reproductive performance were investigated in male (treated for 4 weeks before mating) and female rats (treated for 2 weeks before mating through Postpartum Day 6) that received doses of 300, 820, or 2,240 mg per kg per day. Systemic exposures (AUC0-24 h) to amprenavir in these studies were 3 (males) to 4 (females) times higher than exposures in humans following administration of the MRHD of fosamprenavir alone or similar to those seen in humans following administration of fosamprenavir in combination with ritonavir. Fosamprenavir did not impair mating or fertility of male or female rats and did not affect the development and maturation of sperm from treated rats.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
LEXIVA tablets may be taken with or without food.
Adults should take LEXIVA oral suspension without food. Pediatric patients should take LEXIVA oral suspension with food [see Clinical Pharmacology (12.3)]. If emesis occurs within 30 minutes after dosing, re-dosing of LEXIVA oral suspension should occur.
Higher-than-approved dose combinations of LEXIVA plus ritonavir are not recommended due to an increased risk of transaminase elevations [see Overdosage (10)].
When LEXIVA is used in combination with ritonavir, prescribers should consult the full prescribing information for ritonavir.
2.2 Adults
Therapy-Naive Adults
•
LEXIVA 1,400 mg twice daily (without ritonavir).
•
LEXIVA 1,400 mg once daily plus ritonavir 200 mg once daily.
•
LEXIVA 1,400 mg once daily plus ritonavir 100 mg once daily.
•
Dosing of LEXIVA 1,400 mg once daily plus ritonavir 100 mg once daily is supported by pharmacokinetic data [see Clinical Pharmacology (12.3)].
•
LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily.
•
Dosing of LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily is supported by pharmacokinetic and safety data [see Clinical Pharmacology (12.3)].
Protease Inhibitor-Experienced Adults
•
LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily.
Pregnancy
•
LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily.
•
Dosing of LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily should only be considered in pregnant patients who are already on a stable twice-daily regimen of LEXIVA/ritonavir 700 mg/100 mg prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). Lower exposures of amprenavir were observed during pregnancy; therefore, viral load should be monitored closely to ensure viral suppression is maintained [see Use in Specific Populations (8.1), Clinical Pharmacology (12.3)]. Data regarding use of other regimens of LEXIVA (with or without ritonavir) in pregnancy are not available.
2.3 Pediatric Patients (Aged at Least 4 Weeks to 18 Years)
The recommended dosage of LEXIVA in patients aged at least 4 weeks to 18 years should be calculated based on body weight (kg) and should not exceed the recommended adult dose (Table 1).
Table 1. Twice-Daily Dosage Regimens by Weight for Protease Inhibitor- Naive Pediatric Patients (Aged 4 Weeks and Older) and for Protease Inhibitor- Experienced Pediatric Patients (Aged 6 Months and Older) Using LEXIVA Oral Suspension with Concurrent Ritonavir|
a When dosing with ritonavir, do not exceed the adult dose of LEXIVA 700 mg/ritonavir 100 mg twice-daily dose. | |
|
Weight |
Twice-Daily Dosage Regimen |
|
<11 kg |
LEXIVA 45 mg/kg plus ritonavir 7 mg/kga |
|
11 kg - <15 kg |
LEXIVA 30 mg/kg plus ritonavir 3 mg/kga |
|
15 kg - <20 kg |
LEXIVA 23 mg/kg plus ritonavir 3 mg/kga |
|
≥20 kg |
LEXIVA 18 mg/kg plus ritonavir 3 mg/kga |
Alternatively, protease inhibitor-naive children aged 2 years and older can be administered LEXIVA (without ritonavir) 30 mg per kg twice daily.
LEXIVA should only be administered to infants born at 38 weeks’ gestation or greater and who have attained a postnatal age of 28 days.
For pediatric patients, pharmacokinetic and clinical data:
•
do not support once-daily dosing of LEXIVA alone or in combination with ritonavir [see Clinical Studies (14.3)].
•
do not support administration of LEXIVA alone or in combination with ritonavir for protease inhibitor‑experienced children younger than 6 months [see Clinical Pharmacology (12.3)].
•
do not support twice-daily dosing of LEXIVA without ritonavir in pediatric patients younger than 2 years [see Clinical Pharmacology (12.3)].
Other Dosing Considerations
•
When administered without ritonavir, the adult regimen of LEXIVA tablets 1,400 mg twice daily may be used for pediatric patients weighing at least 47 kg.
•
When administered in combination with ritonavir, LEXIVA tablets may be used for pediatric patients weighing at least 39 kg; ritonavir capsules may be used for pediatric patients weighing at least 33 kg.
2.4 Patients with Hepatic Impairment
See Clinical Pharmacology (12.3).
Mild Hepatic Impairment (Child-Pugh Score Ranging from 5 to 6)
LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 700 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Moderate Hepatic Impairment (Child-Pugh Score Ranging from 7 to 9)
LEXIVA should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 450 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Severe Hepatic Impairment (Child-Pugh Score Ranging from 10 to 15)
LEXIVA should be used with caution at a reduced dosage of 350 mg twice daily without ritonavir (therapy-naive) or 300 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
There are no data to support dosing recommendations for pediatric patients with hepatic impairment.
•
Therapy-Naive Adults: LEXIVA 1,400 mg twice daily; LEXIVA 1,400 mg once daily plus ritonavir 200 mg once daily; LEXIVA 1,400 mg once daily plus ritonavir 100 mg once daily; LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily. (2.2)
•
Protease Inhibitor-Experienced Adults: LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily. (2.2)
•
Pregnant Patients: LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily should only be considered in women who are already on a stable twice-daily regimen of LEXIVA/ritonavir 700 mg/100 mg prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). (2.2)
•
Pediatric Patients (aged at least 4 weeks to 18 years): Dosage should be calculated based on body weight (kg) and should not exceed adult dose. (2.3)
•
Hepatic Impairment: Recommended adjustments for patients with mild, moderate, or severe hepatic impairment. (2.4)
Dosing Considerations
•
LEXIVA tablets may be taken with or without food. (2.1)
•
LEXIVA suspension: Adults should take without food; pediatric patients should take with food. (2.1)
