Redicare Alcohol Prep Pads
3cb7fba1-1d2d-f4da-e063-6394a90a4b21
HUMAN OTC DRUG LABEL
Aug 19, 2025
Redicare LLC
DUNS: 800149346
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Medium Alcohol Prep Pads
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
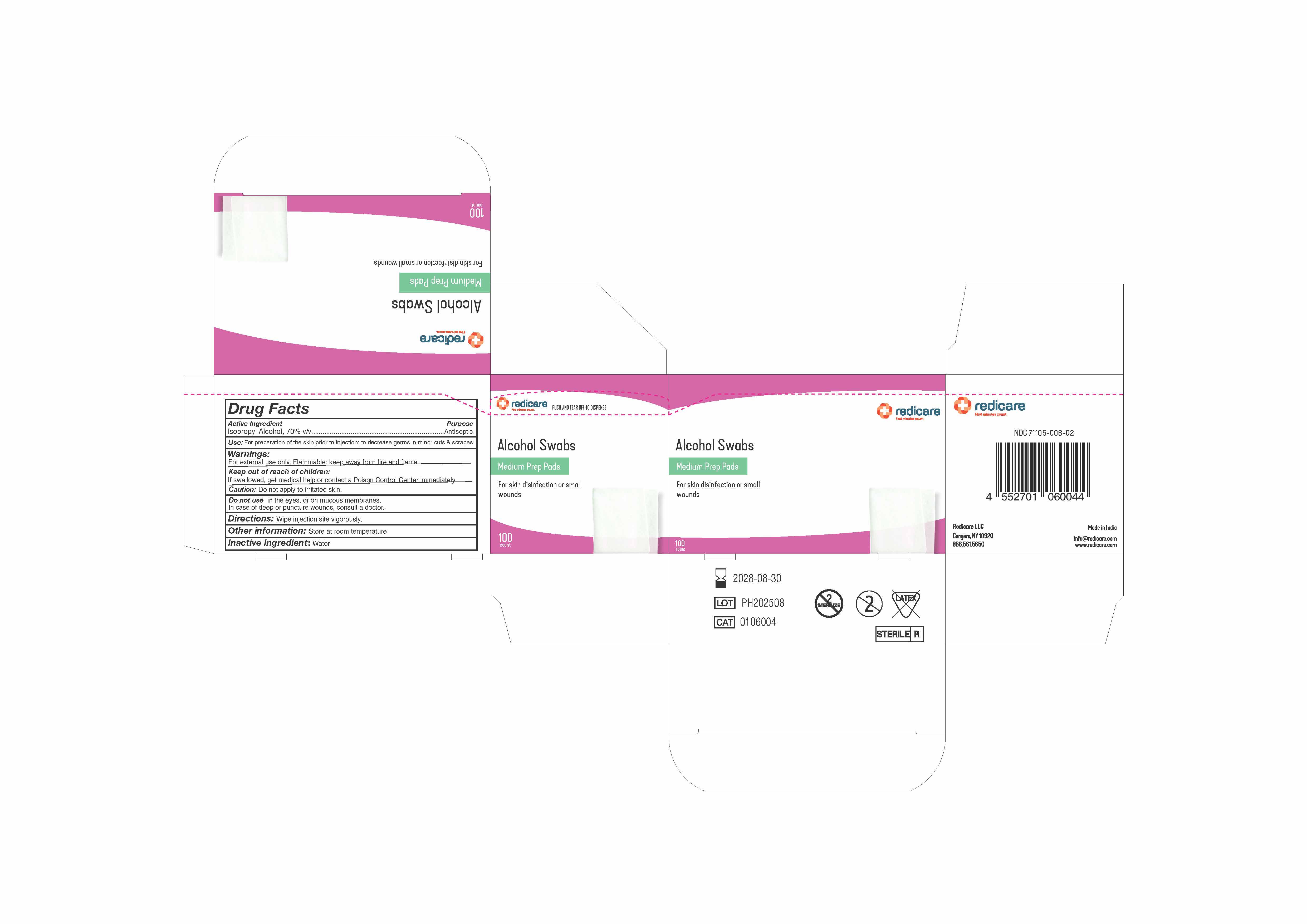
INDICATIONS & USAGE SECTION
Use
For preparation of the skin prior to injection; to decrease germs in minor cuts and scrapes.
INACTIVE INGREDIENT SECTION
Inactive ingredients:
Water
OTHER SAFETY INFORMATION
Other information:
Store at room temperature
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
Isopropyl alcohol 70% v/v
OTC - PURPOSE SECTION
Purpose
Antiseptic
WARNINGS SECTION
Warnings:
For external use only. Flammable; keep away from fire and flame.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Caution:
Do not apply to irritated skin.
Do not use
in the eyes, or on mucous membranes. In case of deep or puncture wounds, consult a doctor.
DOSAGE & ADMINISTRATION SECTION
Directions:
Wipe injection site vigorously.
