Lactulose
LACTULOSE SOLUTION, USP
df62ad54-5aba-4d05-e053-2a95a90aa48d
HUMAN PRESCRIPTION DRUG LABEL
Mar 21, 2023
Xttrium Laboratories, Inc.
DUNS: 007470579
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lactulose
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LACTULOSE 30 mL UNIT DOSE CUP
UNIT DOSE
Delivers 30mL
NDC 0116-4005-30
LACTULOSE SOLUTION, USP
20g/30mL
Rx Only
Xttrium Laboratories, Inc.
4005LACT30LIDA

SPL UNCLASSIFIED SECTION
Rx Only
**Revised:**June 2024
Distributed by:
Xttrium Laboratories, Inc.
1200 E. Business Center Dr.
Mount Prospect, IL 60056
DESCRIPTION SECTION
DESCRIPTION
Lactulose solution is a synthetic disaccharide in solution form for oral administration.Each 15 mL of lactulose contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g or less of other sugars). Also contains water, D&C Yellow No. 10, and FD&C Yellow No. 6. The pH range is 2.5 to 6.5.
Lactulose solution is a colonic acidifier which promotes laxation.
The chemical name for lactulose is 4-O-ß-D-galactopyranosyl-D-fructofuranose. It has the following structural formula:
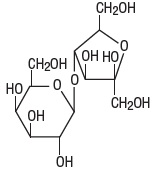
C 12H 220 11
The molecular weight is 342.30. It is freely soluble in water.
SPL PATIENT PACKAGE INSERT SECTION
Prescribing Information Insert