ORPHENADRINE CITRATE
Orphenadrine Citrate Extended-Release TabletsRx Only
7b76c8a7-dd0c-481c-8299-26dfd38ce066
HUMAN PRESCRIPTION DRUG LABEL
Sep 10, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ORPHENADRINE CITRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
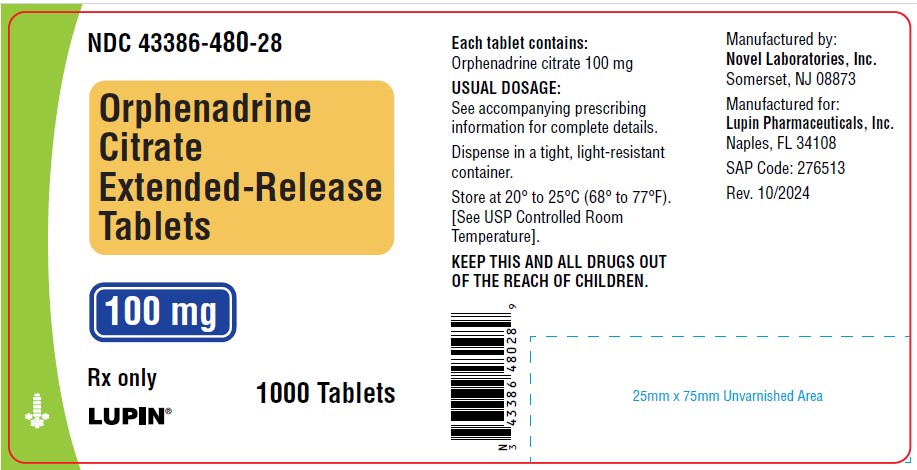
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Orphenadrine Citrate Extended-Release Tablets, 100 mg
Container Label
NDC 43386-480-24
DESCRIPTION SECTION
DESCRIPTION
Orphenadrine citrate is the citrate salt of orphenadrine. It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol. The chemical name of orphenadrine citrate is (±)-N,N-Dimethyl-2-[(o-methyl-α- phenylbenzyl)oxy]ethylamine citrate (1:1) having molecular formula C18H23NO•C6H8O7 and molecular weight of 461.51. It has the following structural formula:
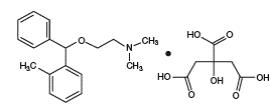
Each tablet for oral administration contains 100 mg orphenadrine citrate. Each Orphenadrine citrate extended- release tablet contains the following inactive ingredients: hydroxypropyl methylcellulose, lactose monohydrate and magnesium stearate.
HOW SUPPLIED SECTION
HOW SUPPLIED
Orphenadrine citrate extended-release tablets 100 mg are round, white to off- white tablets, debossed NL4 on one side and plain on the other side and are supplied as:
NDC 43386-480-24 in bottles of 100 tablets
NDC 43386-480-26 in bottles of 500 tablets
NDC 43386-480-28 in bottles of 1000 tablets
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container.
LUPIN and the  are
registered trademarks of Lupin Pharmaceuticals, Inc.
are
registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured by
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured For
Lupin Pharmaceuticals, Inc.
Naples FL, 34108
SAP Code: 276510
Rev: 10/2024
