Wish ultra winter ice
b4c5487c-1314-4d63-96df-077e72e7ee95
HUMAN OTC DRUG LABEL
Aug 1, 2025
LJ Pharma
DUNS: 914078633
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label
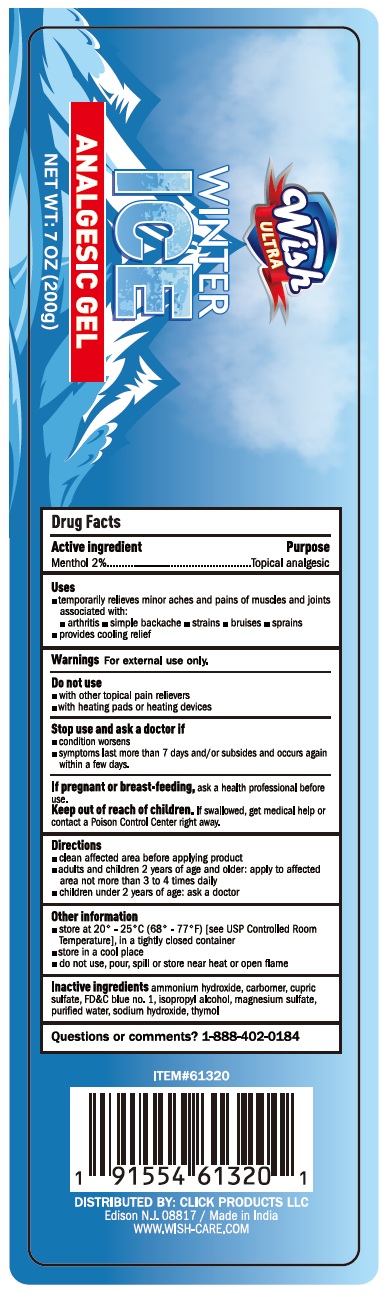
INDICATIONS & USAGE SECTION
Uses
Temporarily relieves minor aches and pains of muscles and joints associated with:
- Arthritis
- Simple backache
- Strains
- Bruises
- Sprains
- Provides cooling relief
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient Purpose
Menthol 2% Topical analgesic
OTC - PURPOSE SECTION
WARNINGS SECTION
Warnings
For external use only
Do not use:
- With other topical pain relievers
- With heating pads or heating devices
Stop use and ask a doctor if:
- Condition worsens
- Symptoms last more than 7 days and/or subside and occur again within a few days
If pregnant or breast-feeding: Ask a health professional before use.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
DOSAGE & ADMINISTRATION SECTION
Directions
- Clean affected area before applying product
- Adults and children 2 years and older: Apply to affected area no more than 3 to 4 times daily
- Children under 2 years of age: Ask a doctor
SPL UNCLASSIFIED SECTION
Questions or Comments? 1-888-402-0184
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Ammonium hydroxide, carbomer, cupric sulfate, FD&C blue no. 1, isopropyl alcohol, magnesium sulfate, purified water, sodium hydroxide, thymol
