Thiola EC
These highlights do not include all the information needed to use THIOLA EC safely and effectively. See full prescribing information for THIOLA EC. THIOLA EC (tiopronin) delayed-release tablets, for oral use Initial U.S. Approval: 1988
20298a52-a194-a161-8632-d84b6f26e23c
HUMAN PRESCRIPTION DRUG LABEL
Sep 30, 2025
Mission Pharmacal Company
DUNS: 008117095
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tiopronin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
tiopronin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
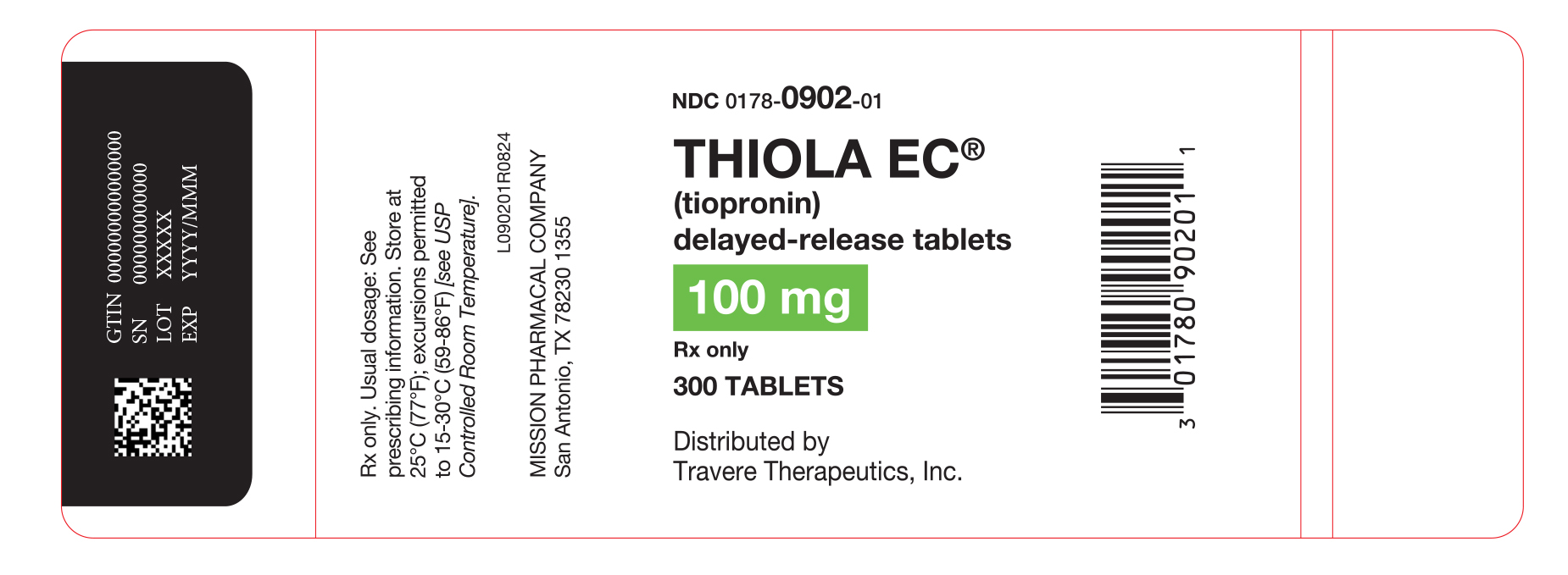
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Thiola EC ®
NDC: 0178-0902-01
Thiola EC ®
NDC: 0178-0901-90

DESCRIPTION SECTION
11 DESCRIPTION
THIOLA EC (tiopronin) delayed-release tablets are a reducing and cystine- binding thiol drug (CBTD) for oral use. Tiopronin is N‑(2‑Mercaptopropionyl) glycine and has the following structure:

Tiopronin has the empirical formula C 5H 9NO 3S and a molecular weight of 163.20. In this drug product tiopronin exists as a dl racemic mixture.
Tiopronin is a white crystalline powder, which is freely soluble in water.
Each THIOLA EC tablet contains 100 or 300 mg of tiopronin. The inactive ingredients in THIOLA EC tablets include lactose monohydrate, hydroxypropyl cellulose, hydroxypropyl cellulose (low substitute), magnesium stearate, hydroxypropyl methylcellulose E5, methacrylic acid: ethyl acrylate copolymer (Eudragit L 100-55), talc, triethyl citrate.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Administration Instructions
For patients who cannot swallow the tablet whole, the THIOLA EC tablets can be
crushed and mixed with applesauce. See Dosage and Administration ( 2.2) for
preparation and administration instructions.
Lactation
Advise women that breastfeeding is not recommended during treatment with
THIOLA EC [see Use in Specific Populations ( 8.2)] .
Manufactured and packaged by Mission Pharmacal Company, San Antonio, TX 78230
1355
Distributed by Travere Therapeutics, Inc., San Diego, CA 92130
Copyright© 2021 Mission Pharmacal Company.
All rights reserved.
L090201R0824
THL_T13768R0924
