Hetastarch in Sodium Chloride
These highlights do not include all the information needed to use 6% Hetastarch in 0.9% Sodium Chloride Injection safely and effectively. See full prescribing information for 6% Hetastarch in 0.9% Sodium Chloride Injection.6% Hetastarch in 0.9% Sodium Chloride Injection, for intravenous useInitial U.S. Approval: 1991
d64f961a-3e1f-4b04-cf83-604474af2775
HUMAN PRESCRIPTION DRUG LABEL
Aug 28, 2023
Hospira, Inc.
DUNS: 141588017
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
HETASTARCH
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
6% Hetastarch in 0.9% Sodium Chloride Injection is a sterile, nonpyrogenic solution for intravenous administration.
Each 100 mL contains:
Hetastarch............................................................................
6 g
Sodium Chloride, USP........................................................
0.9 g
Water for Injection, USP.....................................................
qs
pH adjusted with Sodium Hydroxide, NF if necessary
Concentration of Electrolytes (mEq/L): Sodium (Na+) 154, Chloride (Cl-) 154
(not including ions for pH adjustment).
pH: 5.5 (3.5 to 7.0)
Total osmolar concentration is 308 mOsmol/liter (calc).
Hetastarch is a synthetic colloid derived from a waxy starch composed almost entirely of amylopectin. Hydroxyethyl ether groups are introduced into the glucose units of the starch, and the resultant material is hydrolyzed to yield a product with a molecular weight suitable for use as a plasma volume expander and erythrocyte sedimenting agent. The molar substitution is approximately 0.75 which means hetastarch has an average of approximately 75 hydroxyethyl groups for every 100 glucose units. The weight average molecular weight is approximately 670,000 with a range of 550,000 to 800,000 and with at least 80% of the polymers falling within the range of 20,000 to 2,500,000. Hydroxyethyl groups are attached by ether linkage primarily at C-2 of the glucose unit and to a lesser extent at C-3 and C-6. The polymer resembles glycogen, and the polymerized D-glucose units are joined primarily by α-1,4 linkages with occasional α-1,6 branching linkages. The degree of branching is approximately 1:20 which means that there is one 1–6 branch for every 20 glucose monomer units.
The chemical name for hetastarch is hydroxyethyl starch.
The structural formula is as follows:
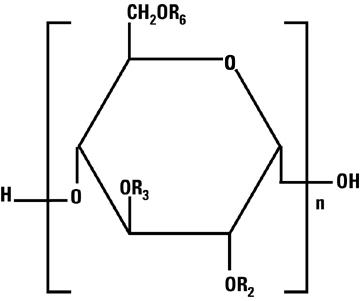
Amylopectin derivative in which R2, R3, and R6 are H or CH2CH2OH, or R6 is a branching point in the starch polymer connected through a 1-6 linkage to additional alpha-D-glucopyranosyl units.
Hetastarch is an artificial colloid pharmacologically classified as a plasma volume expander; 0.9% Sodium Chloride Injection is a fluid and electrolyte replenisher.
6% Hetastarch in 0.9% Sodium Chloride Injection is a clear, pale yellow to amber solution. Exposure to prolonged adverse storage conditions may result in a change to a turbid deep brown or the formation of a crystalline precipitate. Do not use the solution if these conditions are evident.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly.
Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
