AMANTADINE HYDROCHLORIDE
Amantadine Hydrochloride Oral Solution USP 50 mg/5 mL
6311b46b-23cc-40c1-93c2-f7c92606386f
HUMAN PRESCRIPTION DRUG LABEL
Mar 4, 2022
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
AMANTADINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Cup Label


ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The adverse reactions reported most frequently at the recommended dose of amantadine hydrochloride (5 to 10%) are: nausea, dizziness (lightheadedness), and insomnia.
Less frequently (1 to 5%) reported adverse reactions are: depression, anxiety and irritability, hallucinations, confusion, anorexia, dry mouth, constipation, ataxia, livedo reticularis, peripheral edema, orthostatic hypotension, headache, somnolence, nervousness, dream abnormality, agitation, dry nose, diarrhea and fatigue.
Infrequently (0.1 to 1%) occurring adverse reactions are: congestive heart failure, psychosis, urinary retention, dyspnea, skin rash, vomiting, weakness, slurred speech, euphoria, thinking abnormality, amnesia, hyperkinesia, hypertension, decreased libido, and visual disturbance, including punctate subepithelial or other corneal opacity, corneal edema, decreased visual acuity, sensitivity to light, and optic nerve palsy.
Rare (less than 0.1%) occurring adverse reactions are: instances of convulsion, leukopenia, neutropenia, eczematoid dermatitis, oculogyric episodes, suicidal attempt, suicide, and suicidal ideation ( seeWARNINGS).
Other adverse reactions reported during postmarketing experience with amantadine hydrochloride usage include:
Nervous System/Psychiatric
coma, stupor, delirium, hypokinesia, hypertonia, delusions, aggressive behavior, paranoid reaction, manic reaction, involuntary muscle contractions, gait abnormalities, paresthesia, EEG changes, and tremor. Abrupt discontinuation may also precipitate delirium, agitation, delusions, hallucinations, paranoid reaction, stupor, anxiety, depression and slurred speech;
Cardiovascular
cardiac arrest, arrhythmias including malignant arrhythmias, hypotension, and tachycardia;
Respiratory
acute respiratory failure, pulmonary edema, and tachypnea;
Gastrointestinal
dysphagia;
Hematologic
leukocytosis and agranulocytosis;
Special Senses
keratitis and mydriasis;
Skin and Appendages
pruritus and diaphoresis;
Miscellaneous
neuroleptic malignant syndrome ( seeWARNINGS), allergic reactions including anaphylactic reactions, edema and fever.
Laboratory Tests
elevated: CPK, BUN, serum creatinine, alkaline phosphatase, LDH, bilirubin, GGT, SGOT, and SGPT.
DESCRIPTION SECTION
DESCRIPTION
Amantadine Hydrochloride Oral Solution USP is designated generically as amantadine hydrochloride and chemically as 1-adamantanamine hydrochloride.
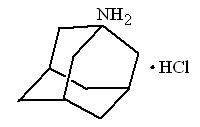
Amantadine hydrochloride is a stable white or nearly white crystalline powder, freely soluble in water and soluble in alcohol and in chloroform.
Amantadine hydrochloride has pharmacological actions as both an anti-Parkinson and an antiviral drug.
Amantadine Hydrochloride Oral Solution USP contains 50 mg of amantadine hydrochloride per 5 mL and has the following inactive ingredients: artificial raspberry flavor, citric acid, methylparaben, propylene glycol, propylparaben, purified water, sorbitol solution. May contain sodium hydroxide to adjust pH to approximately 2.3.
WARNINGS SECTION
WARNINGS
Deaths
Deaths have been reported from overdose with amantadine hydrochloride. The lowest reported acute lethal dose was 1 gram. Acute toxicity may be attributable to the anticholinergic effects of amantadine hydrochloride. Drug overdose has resulted in cardiac, respiratory, renal or central nervous system toxicity. Cardiac dysfunction includes arrhythmia, tachycardia and hypertension ( seeOVERDOSAGE). Deaths due to drug accumulation (overdose) have been reported in patients with renal impairment, who were prescribed higher than recommended doses of amantadine hydrochloride for their level of renal function ( seeDOSAGE AND ADMINISTRATION; Dosage of Impaired Renal Functionand OVERDOSAGE).
Suicide Attempts
Suicide attempts, some of which have been fatal, have been reported in patients treated with amantadine hydrochloride, many of whom received short courses for influenza treatment or prophylaxis. The incidence of suicide attempts is not known and the pathophysiologic mechanism is not understood. Suicide attempts and suicidal ideation have been reported in patients with and without prior history of psychiatric illness. Amantadine hydrochloride can exacerbate mental problems in patients with a history of psychiatric disorders or substance abuse. Patients who attempt suicide may exhibit abnormal mental states which include disorientation, confusion, depression, personality changes, agitation, aggressive behavior, hallucinations, paranoia, other psychotic reactions and somnolence or insomnia. Because of the possibility of serious adverse effects, caution should be observed when prescribing amantadine hydrochloride to patients being treated with drugs having CNS effects, or for whom the potential risks outweigh the benefit of treatment.
CNS Effects
Patients with a history of epilepsy or other "seizures" should be observed closely for possible increased seizure activity.
Patients receiving amantadine hydrochloride who note central nervous system effects or blurring of vision should be cautioned against driving or working in situations where alertness and adequate motor coordination are important.
Other
Patients with a history of congestive heart failure or peripheral edema should be followed closely as there are patients who developed congestive heart failure while receiving amantadine hydrochloride.
Patients with Parkinson's disease improving on amantadine hydrochloride should resume normal activities gradually and cautiously, consistent with other medical considerations, such as the presence of osteoporosis or phlebothrombosis.
Because amantadine hydrochloride has anticholinergic effects and may cause mydriasis, it should not be given to patients with untreated angle closure glaucoma.
