NP Thyroid
3c270b9a-05c2-6a30-e063-6394a90a2844
HUMAN OTC DRUG LABEL
Aug 12, 2025
Calvin Scott & Co Inc.
DUNS: 073404626
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Thyroid tablets
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
17224-511-60
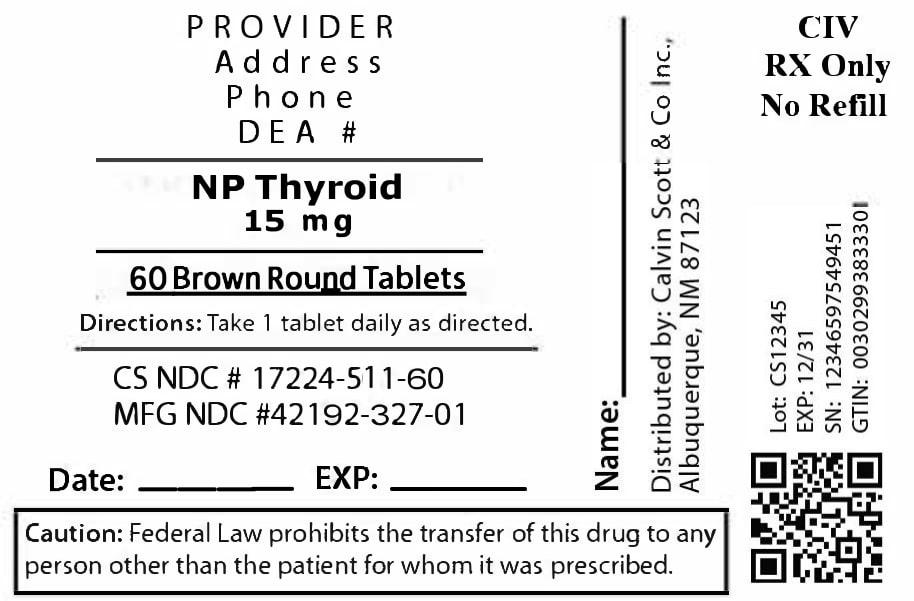 Package
label for NP Thyroid 15 mg tablets (60 tablets per bottle). Contains product
name, dosage strength, NDC 17224-511-60, manufacturer/repacker name, and usage
instructions as printed on the label.
Package
label for NP Thyroid 15 mg tablets (60 tablets per bottle). Contains product
name, dosage strength, NDC 17224-511-60, manufacturer/repacker name, and usage
instructions as printed on the label.
INDICATIONS & USAGE SECTION
Used as a thyroid hormone replacement for hypothyroidism.
OTC - PURPOSE SECTION
Thyroid hormone replacement
OTC - ACTIVE INGREDIENT SECTION
Thyroid 15 mg
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of accidental overdose, contact a doctor or Poison Control Center immediately.
DOSAGE & ADMINISTRATION SECTION
Take 1 tablet daily on an empty stomach or as directed.
WARNINGS SECTION
Do not exceed recommended dose. Keep out of reach of children.
INACTIVE INGREDIENT SECTION
Calcium stearate, dextrose, maltodextrin, mineral oil, modified wheat starch
