Rocuronium Bromide
These highlights do not include all the information needed to use ROCURONIUM BROMIDE INJECTION safely and effectively. See full prescribing information for ROCURONIUM BROMIDE INJECTION. ROCURONIUM BROMIDE injection, solution for intravenous use Initial U.S. Approval: 1994
116385ea-69fc-4f44-b463-6210291032df
HUMAN PRESCRIPTION DRUG LABEL
Aug 5, 2023
Eugia US LLC
DUNS: 968961354
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rocuronium Bromide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Rocuronium Bromide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - Peel-off Label 10 mg/mL
Rx only Peel off & apply to syringe
**Rocuronium Bromide Injection
****** For Intravenous Use Only 10 mg/10 mL
****Date:_________ Time: _________ By: _________

DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Antibiotics
Drugs which may enhance the neuromuscular blocking action of nondepolarizing agents such as rocuronium bromide include certain antibiotics (e.g., aminoglycosides; vancomycin; tetracyclines; bacitracin; polymyxins; colistin; and sodium colistimethate). If these antibiotics are used in conjunction with rocuronium bromide, prolongation of neuromuscular block may occur.
7.2 Anticonvulsants
In 2 of 4 patients receiving chronic anticonvulsant therapy, apparent resistance to the effects of rocuronium bromide was observed in the form of diminished magnitude of neuromuscular block, or shortened clinical duration. As with other nondepolarizing neuromuscular blocking drugs, if rocuronium bromide is administered to patients chronically receiving anticonvulsant agents such as carbamazepine or phenytoin, shorter durations of neuromuscular block may occur and infusion rates may be higher due to the development of resistance to nondepolarizing muscle relaxants. While the mechanism for development of this resistance is not known, receptor up-regulation may be a contributing factor [see Warnings and Precautions (5.10)].
7.3 Inhalation Anesthetics
Use of inhalation anesthetics has been shown to enhance the activity of other neuromuscular blocking agents (enflurane > isoflurane > halothane).
Isoflurane and enflurane may also prolong the duration of action of initial and maintenance doses of rocuronium bromide and decrease the average infusion requirement of rocuronium bromide by 40% compared to opioid/nitrous oxide/oxygen anesthesia. No definite interaction between rocuronium bromide and halothane has been demonstrated. In one study, use of enflurane in 10 patients resulted in a 20% increase in mean clinical duration of the initial intubating dose, and a 37% increase in the duration of subsequent maintenance doses, when compared in the same study to 10 patients under opioid/nitrous oxide/oxygen anesthesia. The clinical duration of initial doses of rocuronium bromide of 0.57 to 0.85 mg/kg under enflurane or isoflurane anesthesia, as used clinically, was increased by 11% and 23%, respectively. The duration of maintenance doses was affected to a greater extent, increasing by 30% to 50% under either enflurane or isoflurane anesthesia.
Potentiation by these agents is also observed with respect to the infusion rates of rocuronium bromide required to maintain approximately 95% neuromuscular block. Under isoflurane and enflurane anesthesia, the infusion rates are decreased by approximately 40% compared to opioid/nitrous oxide/oxygen anesthesia. The median spontaneous recovery time (from 25% to 75% of control T1) is not affected by halothane, but is prolonged by enflurane (15% longer) and isoflurane (62% longer). Reversal-induced recovery of rocuronium bromide neuromuscular block is minimally affected by anesthetic technique [see Dosage and Administration (2.6) and Warnings and Precautions (5.10)].
7.4 Lithium Carbonate
Lithium has been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.5 Local Anesthetics
Local anesthetics have been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.6 Magnesium
Magnesium salts administered for the management of toxemia of pregnancy may enhance neuromuscular blockade [see Warnings and Precautions (5.10)].
7.7 Nondepolarizing Muscle Relaxants
There are no controlled studies documenting the use of rocuronium bromide before or after other nondepolarizing muscle relaxants. Interactions have been observed when other nondepolarizing muscle relaxants have been administered in succession.
7.8 Procainamide
Procainamide has been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.9 Propofol
The use of propofol for induction and maintenance of anesthesia does not alter the clinical duration or recovery characteristics following recommended doses of rocuronium bromide.
7.10 Quinidine
Injection of quinidine during recovery from use of muscle relaxants is associated with recurrent paralysis. This possibility must also be considered for rocuronium bromide [see Warnings and Precautions (5.10)].
7.11 Succinylcholine
The use of rocuronium bromide before succinylcholine, for the purpose of attenuating some of the side effects of succinylcholine, has not been studied.
If rocuronium bromide is administered following administration of succinylcholine, it should not be given until recovery from succinylcholine has been observed. The median duration of action of rocuronium bromide 0.6 mg/kg administered after a 1 mg/kg dose of succinylcholine when T1 returned to 75% of control was 36 minutes (range: 14 to 57, n = 12) vs. 28 minutes (range: 17 to 51, n = 12) without succinylcholine.
- Succinylcholine: Use before succinylcholine has not been studied. (7.11)
- Nondepolarizing muscle relaxants: Interactions have been observed. (7.7)
- Enhanced rocuronium bromide activity possible: Inhalation anesthetics (7.3), certain antibiotics (7.1), quinidine (7.10), magnesium (7.6), lithium (7.4), local anesthetics (7.5), procainamide (7.8)
- Reduced rocuronium bromide activity possible: Anticonvulsants. (7.2)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rocuronium bromide is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration. It acts by competing for cholinergic receptors at the motor end-plate. This action is antagonized by acetylcholinesterase inhibitors, such as neostigmine and edrophonium.
12.2 Pharmacodynamics
The ED95 (dose required to produce 95% suppression of the first [T1] mechanomyographic [MMG] response of the adductor pollicis muscle [thumb] to indirect supramaximal train-of-four stimulation of the ulnar nerve) during opioid/nitrous oxide/oxygen anesthesia is approximately 0.3 mg/kg. Patient variability around the ED95 dose suggests that 50% of patients will exhibit T1 depression of 91% to 97%.
** Table 4** presents intubating conditions in patients with intubation initiated at 60 to 70 seconds.
Table 4: Percent of Excellent or Good Intubating Conditions and Median (Range) Time to Completion of Intubation in Patients with Intubation Initiated at 60 to 70 Seconds
| ||
|
Rocuronium Bromide Dose (mg/kg) |
Percent of Patients with Excellent or Good |
Time to Completion |
|
Adults* 18 to 64 yrs |
86% |
1.6 (1 to 7) |
|
Infants† 3 mo to 1 yr |
100% 100% |
1 (1 to 1.5) 1 (0.5 to 2.3) |
Table 5 presents the time to onset and clinical duration for the initial dose of rocuronium bromide injection under opioid/nitrous oxide/oxygen anesthesia in adults and geriatric patients, and under halothane anesthesia in pediatric patients.
Table 5: Median (Range) Time to Onset and Clinical Duration Following Initial (Intubating) Dose During Opioid/Nitrous Oxide/Oxygen Anesthesia (Adults) and Halothane Anesthesia (Pediatric Patients)|
n = the number of patients who had time to maximum block recorded. | |||
|
Rocuronium Bromide Dose (mg/kg) |
Time to |
Time to |
Clinical |
|
Adults 18 to 64 yrs |
1.3 (0.8 to 6.2) |
3 (1.3 to 8.2) |
22 (12 to 31) |
|
Geriatric ≥65 yrs |
2.3 (1 to 8.3) |
3.7 (1.3 to 11.3) |
46 (22 to 73) |
|
Infants 3 mo to 1 yr |
— |
0.8 (0.3 to 3) |
41 (24 to 68) |
|
Pediatric 1 to 12 yrs |
0.8 (0.4 to 2) |
1 (0.5 to 3.3) |
26 (17 to 39) |
Table 6presents the time to onset and clinical duration for the initial dose of rocuronium bromide injection under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia in pediatric patients.
Table 6: Median (Range) Time to Onset and Clinical Duration Following Initial (Intubating) Dose During Sevoflurane (induction) and Isoflurane/Nitrous Oxide (maintenance) Anesthesia (Pediatric Patients)|
n = the number of patients with the highest number of observations for time to maximum block or reappearance T3. | ||
|
Rocuronium Bromide Dose (mg/kg) Administered Over 5 sec |
Time to |
Time to |
|
Neonates birth to <28 days |
1.1 (0.6 to 2.2) |
40.3 (32.5 to 62.6) |
|
Infants 28 days to ≤3 mo |
0.5 (0.4 to 1.3) |
49.1 (13.5 to 79.9) |
|
Toddlers >3 mo to ≤2 yrs |
0.8 (0.3 to 1.9) |
39.2 (16.9 to 59.4) |
|
Children >2 yrs to ≤11 yrs |
0.9 (0.4 to 1.9) |
21.5 (17.5 to 38) |
|
Adolescents >11 to ≤17 yrs |
1 (0.5 to 1.7) |
37.5 (18.3 to 65.7) |
The time to 80% or greater block and clinical duration as a function of dose are presented inFigures 1 and2.
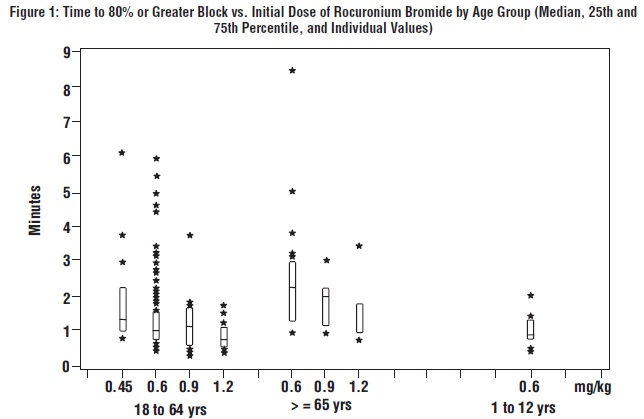
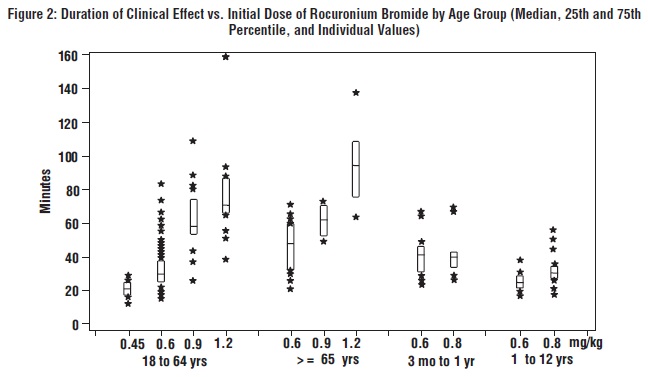
The clinical durations for the first 5 maintenance doses, in patients receiving 5 or more maintenance doses are represented inFigure 3[see Dosage and Administration (2.4)].

Once spontaneous recovery has reached 25% of control T1, the neuromuscular block produced by rocuronium bromide is readily reversed with anticholinesterase agents, e.g., edrophonium or neostigmine.
The median spontaneous recovery from 25% to 75% T1 was 13 minutes in adult patients. When neuromuscular block was reversed in 36 adults at a T1 of 22% to 27%, recovery to a T1 of 89 (50 to 132)% and T4/T1 of 69 (38 to 92)% was achieved within 5 minutes. Only 5 of 320 adults reversed received an additional dose of reversal agent. The median (range) dose of neostigmine was 0.04 (0.01 to 0.09) mg/kg and the median (range) dose of edrophonium was 0.5 (0.3 to 1) mg/kg.
In geriatric patients (n = 51) reversed with neostigmine, the median T4/T1 increased from 40% to 88% in 5 minutes.
In clinical trials with halothane, pediatric patients (n = 27) who received 0.5 mg/kg edrophonium had increases in the median T4/T1 from 37% at reversal to 93% after 2 minutes. Pediatric patients (n = 58) who received 1 mg/kg edrophonium had increases in the median T4/T1 from 72% at reversal to 100% after 2 minutes. Infants (n = 10) who were reversed with 0.03 mg/kg neostigmine recovered from 25% to 75% T1 within 4 minutes.
There were no reports of less than satisfactory clinical recovery of neuromuscular function.
The neuromuscular blocking action of rocuronium bromide may be enhanced in the presence of potent inhalation anesthetics [see Drug Interactions (7.3)].
Hemodynamics
There were no dose-related effects on the incidence of changes from baseline (30% or greater) in mean arterial blood pressure (MAP) or heart rate associated with rocuronium bromide administration over the dose range of 0.12 to 1.2 mg/kg (4 x ED95) within 5 minutes after rocuronium bromide administration and prior to intubation. Increases or decreases in MAP were observed in 2% to 5% of geriatric and other adult patients, and in about 1% of pediatric patients. Heart rate changes (30% or greater) occurred in 0% to 2% of geriatric and other adult patients. Tachycardia (30% or greater) occurred in 12 of 127 pediatric patients. Most of the pediatric patients developing tachycardia were from a single study where the patients were anesthetized with halothane and who did not receive atropine for induction [see Clinical Studies (14.3)]. In U.S. studies, laryngoscopy and tracheal intubation following rocuronium bromide administration were accompanied by transient tachycardia (30% or greater increases) in about one-third of adult patients under opioid/nitrous oxide/oxygen anesthesia. Animal studies have indicated that the ratio of vagal:neuromuscular block following rocuronium bromide administration is less than vecuronium but greater than pancuronium. The tachycardia observed in some patients may result from this vagal blocking activity.
Histamine Release
In studies of histamine release, clinically significant concentrations of plasma histamine occurred in 1 of 88 patients. Clinical signs of histamine release (flushing, rash, or bronchospasm) associated with the administration of rocuronium bromide were assessed in clinical trials and reported in 9 of 1137 (0.8%) patients.
12.3 Pharmacokinetics
Adult and Geriatric Patients
In an effort to maximize the information gathered in the in vivo pharmacokinetic studies, the data from the studies was used to develop population estimates of the parameters for the subpopulations represented (e.g., geriatric, pediatric, renal, and hepatic impairment). These population- based estimates and a measure of the estimate variability are contained in the following section.
Following intravenous administration of rocuronium bromide injection, plasma levels of rocuronium follow a three-compartment open model. The rapid distribution half-life is 1 to 2 minutes and the slower distribution half-life is 14 to 18 minutes. Rocuronium is approximately 30% bound to human plasma proteins. In geriatric and other adult surgical patients undergoing either opioid/nitrous oxide/oxygen or inhalational anesthesia, the observed pharmacokinetic profile was essentially unchanged [see Dosage and Administration (2.6)].
Table 7: Mean (SD) Pharmacokinetic Parameters in Adults (n = 22; ages 27 to 58 yrs) and Geriatric (n = 20; 65 yrs or greater) During Opioid/Nitrous Oxide/Oxygen Anesthesia|
** PK Parameters** |
Adults |
Geriatrics |
|
Clearance (L/kg/hr) |
0.25 (0.08) |
0.21 (0.06) |
|
Volume of Distribution at Steady State (L/kg) |
0.25 (0.04) |
0.22 (0.03) |
|
t1/2 β Elimination (hr) |
1.4 (0.4) |
1.5 (0.4) |
In general, studies with normal adult subjects did not reveal any differences in the pharmacokinetics of rocuronium due to gender.
Studies of distribution, metabolism, and excretion in cats and dogs indicate that rocuronium is eliminated primarily by the liver. The rocuronium analog 17-desacetyl-rocuronium, a metabolite, has been rarely observed in the plasma or urine of humans administered single doses of 0.5 to 1 mg/kg with or without a subsequent infusion (for up to 12 hr) of rocuronium. In the cat, 17-desacetyl-rocuronium has approximately one-twentieth the neuromuscular blocking potency of rocuronium. The effects of renal failure and hepatic disease on the pharmacokinetics and pharmacodynamics of rocuronium in humans are consistent with these findings.
In general, patients undergoing cadaver kidney transplant have a small reduction in clearance which is offset pharmacokinetically by a corresponding increase in volume, such that the net effect is an unchanged plasma half-life. Patients with demonstrated liver cirrhosis have a marked increase in their volume of distribution resulting in a plasma half-life approximately twice that of patients with normal hepatic function.Table 8 shows the pharmacokinetic parameters in subjects with either impaired renal or hepatic function.
Table 8: Mean (SD) Pharmacokinetic Parameters in Adults with Normal Renal and Hepatic Function (n = 10, ages 23 to 65), Renal Transplant Patients (n = 10, ages 21 to 45), and Hepatic Dysfunction Patients (n = 9, ages 31 to 67) During Isoflurane Anesthesia
| |||
|
** PK Parameters** |
Normal Renal and Hepatic Function |
Renal Transplant Patients |
Hepatic Dysfunction Patients |
|
Clearance (L/kg/hr) |
0.16 (0.05)* |
0.13 (0.04) |
0.13 (0.06) |
|
Volume of Distribution at Steady State (L/kg) |
0.26 (0.03) |
0.34 (0.11) |
0.53 (0.14) |
|
t1/2 β Elimination (hr) |
2.4 (0.8)* |
2.4 (1.1) |
4.3 (2.6) |
The net result of these findings is that subjects with renal failure have clinical durations that are similar to but somewhat more variable than the duration that one would expect in subjects with normal renal function. Hepatically impaired patients, due to the large increase in volume, may demonstrate clinical durations approaching 1.5 times that of subjects with normal hepatic function. In both populations the clinician should individualize the dose to the needs of the patient [see Dosage and Administration (2.6)].
Tissue redistribution accounts for most (about 80%) of the initial amount of rocuronium administered. As tissue compartments fill with continued dosing (4 to 8 hours), less drug is redistributed away from the site of action and, for an infusion-only dose, the rate to maintain neuromuscular blockade falls to about 20% of the initial infusion rate. The use of a loading dose and a smaller infusion rate reduces the need for adjustment of dose.
Pediatric Patients
Under halothane anesthesia, the clinical duration of effects of rocuronium bromide did not vary with age in patients 4 months to 8 years of age. The terminal half-life and other pharmacokinetic parameters of rocuronium in these pediatric patients are presented inTable 9.
Table 9: Mean (SD) Pharmacokinetic Parameters of Rocuronium in Pediatric Patients (ages 3 to less than 12 mos, n = 6; 1 to less than 3 yrs, n = 5; 3 to less than 8 yrs, n = 7) During Halothane Anesthesia
|
** PK Parameters** |
Patient Age Range | ||
|
3 to <12 mos |
1 to <3 yrs |
3 to <8 yrs | |
|
Clearance (L/kg/hr) |
0.35 (0.08) |
0.32 (0.07) |
0.44 (0.16) |
|
Volume of Distribution at Steady State (L/kg) |
0.30 (0.04) |
0.26 (0.06) |
0.21 (0.03) |
|
t1/2 β Elimination (hr) |
1.3 (0.5) |
1.1 (0.7) |
0.8 (0.3) |
Pharmacokinetics of rocuronium bromide were evaluated using a population analysis of the pooled pharmacokinetic datasets from 2 trials under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia. All pharmacokinetic parameters were found to be linearly proportional to body weight. In patients under the age of 18 years clearance (CL) and volume of distribution (Vss) increase with bodyweight (kg) and age (years). As a result the terminal half-life of rocuronium bromide decreases with increasing age from 1.1 hour to 0.7 to 0.8 hour.Table 10presents the pharmacokinetic parameters in the different age groups in the studies with sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia.
Table 10: Mean (SD) Pharmacokinetic Parameters of Rocuronium in Pediatric Patients During Sevoflurane (induction) and Isoflurane/Nitrous Oxide (maintenance) Anesthesia|
** PK Parameters** |
Patient Age Range | ||||
|
Birth to <28 days |
28 days to ≤3 mos |
3 mos to |
2 to ≤11 yrs |
11 to ≤17 yrs | |
|
CL (L/kg/hr) |
0.31 (0.07) |
0.30 (0.08) |
0.33 (0.10) |
0.35 (0.09) |
0.29 (0.14) |
|
Volume of Distribution (L/kg) |
0.42 (0.06) |
0.31 (0.03) |
0.23 (0.03) |
0.18 (0.02) |
0.18 (0.01) |
|
t1/2 β (hr) |
1.1 (0.2) |
0.9 (0.3) |
0.8 (0.2) |
0.7 (0.2) |
0.8 (0.3) |
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed with rocuronium bromide to evaluate carcinogenic potential or impairment of fertility. Mutagenicity studies (Ames test, analysis of chromosomal aberrations in mammalian cells, and micronucleus test) conducted with rocuronium bromide did not suggest mutagenic potential.
